The last few decades of the 20th century witnessed growing concerns over the impact of industry on the global environment. These concerns included acid rain, increasing levels of greenhouse gases, fertilizers in streams and rivers, polluted city atmospheres, and a hole in the ozone layer. Some of these are directly attributable to power generation and transport, which have caused major problems and lie outside the direct influence of the chemical industry.
However, in spite of an enormous investment over the last 50 years to ensure that the production of chemicals does not have a malign effect on the environment, many of the public, the very consumers of the products, still associate the chemical industry with the worst sorts of pollution.
There is no doubt that there are still mistakes and these are generally well publicised in the media but overall there have been significant changes in the operation of the chemical industry that are designed to reduce the impact on the environment.
|
|||||
The acceptance that the chemical industry must not adversely affect the environment for future generations has been the driving force behind the development of green chemistry. This is not a separate branch of chemistry, but an approach that permeates every stage of process development.
The aspiration can be summed up in one word: sustainability. Sustainable development and manufacture meets the needs of the present without compromising the ability of future generations to meet their own needs. The problems it aims to address are:
- the depletion of finite oil, gas and mineral resources
- the production of waste, some of it harmful to living organisms
- reagents and processes that present a risk to human health and the environment
- products, which when disposed of, do not degrade easily.
The principles of green chemistry
|
1. Prevention |
|
2. Atom Economy |
|
3. Less Hazardous Chemical Synthesis |
|
4. Designing Safer Chemicals |
|
5. Safer Solvents and Auxiliaries |
|
6. Design for Energy Efficiency |
|
7. Use of Renewable Feedstocks |
|
8. Reduce Derivatives Unnecessary derivatization (use of blocking groups, protection/de-protection, and temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste. |
|
9. Catalysis Catalytic reagents (as selective as possible) are superior to stoichiometric reagents. |
|
10. Design for Degradation |
|
11. Real-time Analysis for Pollution Prevention |
|
12. Inherently Safer Chemistry for Safer Prevention |
| Table 1 The twelve principles of green chemistry. By kind permission of the U.S. Environmental Protection Agency. |
In the early 1990s, the term green chemistry was introduced by the Environmental Protection Agency, an agency of the US Government. The EPA produced a set of 12 principles to guide the chemical industry (Table 1) and in this unit some of these principles will be explained, using wherever possible examples taken from subsequent units dealing with the manufacture of chemicals. These examples illustrate how the search for ways of meeting these principles is a continuing activity. In many instances, changes which reduce the environmental impact of a process also lead to an increase in the profitability of the process. For example, if a new catalyst is developed that reduces the operating temperature and pressure for the process, less energy is consumed which is good both for the environment and for the company.
Prevention
Manufacturers try to generate as little waste as possible, through reaction choice, process design and recycling. Industry aims to use chemical reactions and processes that make the most effective use of available resources and generate the smallest possible amount of waste material. But can prevention be assessed quantitatively?
One way of measuring the efficiency of a process is to calculate the yield, which compares the expected product quantity with the actual amount obtained (although some potential product may be 'lost' as a result of competing reactions).
An example is the manufacture of phenol. It used to be made from benzene using sulfuric acid and sodium hydroxide in a multi-stage process, which, overall, can be expressed as:

The chemical equation shows that 1 mol of benzene (78 g) should yield 1 mol of phenol (94 g). In practice, the quantity of phenol produced is found to be about 77 g, giving a yield of 82%, which may be regarded as quite good.
(Yield % = mass produced / mass expected x 100 %)
However, the calculation obscures the fact that the reaction also generates 1 mol (126 g) of sodium sulfite for each mole of phenol produced. This may be acceptable if there is enough demand for sodium sulfite, but if not, it presents a serious problem of waste management and adds significantly to costs, meaning that this may not be the most suitable reaction for manufacturing phenol.
Atom economy
As an alternative measure to yield, the concept of atom economy is used, considered to be one of the key ideas behind the concepts of green chemistry. This expresses the proportion of reactant atoms that end up in a useful product, measuring the number of atoms of the starting materials that end up as products that are wanted and the number that end up as waste. Atom economy is defined as
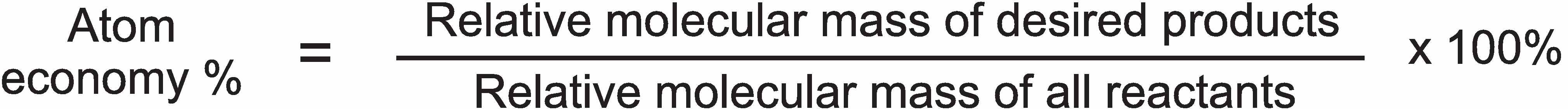
The nearer the value is to 100, the less the waste will be.
This calculation gives an atom economy of only 37% for the manufacture of phenol by the old process (assuming sodium sulfite is waste), a clear indicator that it was wise to develop an alternative process.
The manufacture of phenol is generally from benzene and propene, again in consecutive processes which can be expressed, overall, as:

The co-product is propanone which is a valuable chemical and so the atom economy for this process can be regarded as 100%.
Some reactions that have 100% atom economy have poor yields and so it is necessary to consider both measures of efficiency, yield and atom economy. Atom economy is determined in the planning stage, by calculation, while yield can only be found experimentally.
In organic chemistry, some types of reaction have inherently better atom economies. Addition, condensation and rearrangement reactions will generally have higher atom economies than either elimination or substitution. For example the addition of chlorine to ethene, to form 1,2-dichloroethane (an important reaction in the manufacture of poly(chloroethene) (PVC)) has an atom economy of 100%:

However if the product is hydrolyzed, the atom economy falls:

The first is an addition reaction; the latter is a substitution reaction.
Less hazardous chemical synthesis
The family of polycarbonates contains very important polymers which are used where high optical properties combined with strength are needed. The polycarbonate most used is manufactured from bisphenol A, whose structure is:
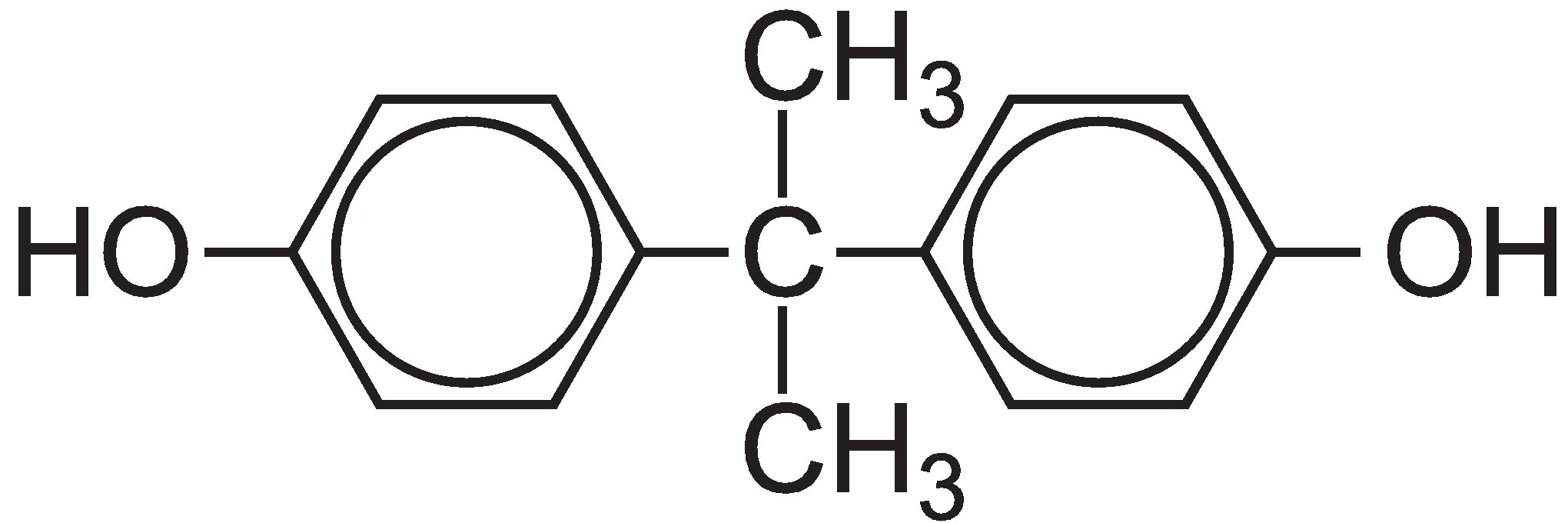
The polycarbonate is manufactured by a condensation reaction between bisphenol A and either carbonyl chloride or diphenyl carbonate.
Carbonyl chloride is a very poisonous gas, manufactured from other hazardous gases, carbon monoxide and chlorine:
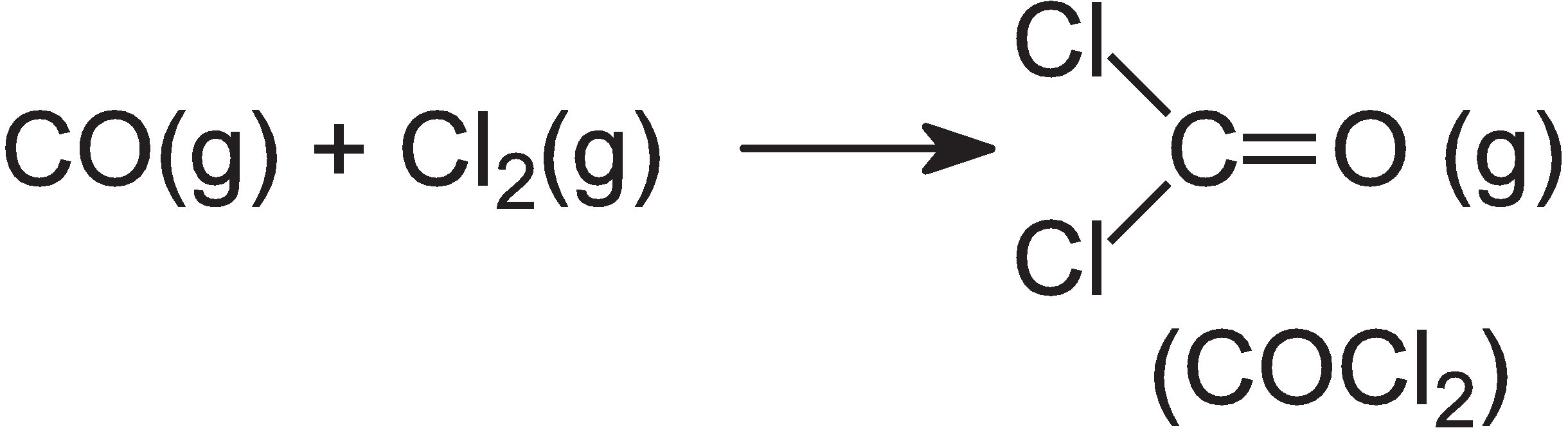
On the other hand, diphenyl carbonate is produced from dimethyl carbonate, which is readily manufactured from methanol, carbon monoxide and oxygen in the liquid phase, in presence of copper(II) chloride, CuCl2:
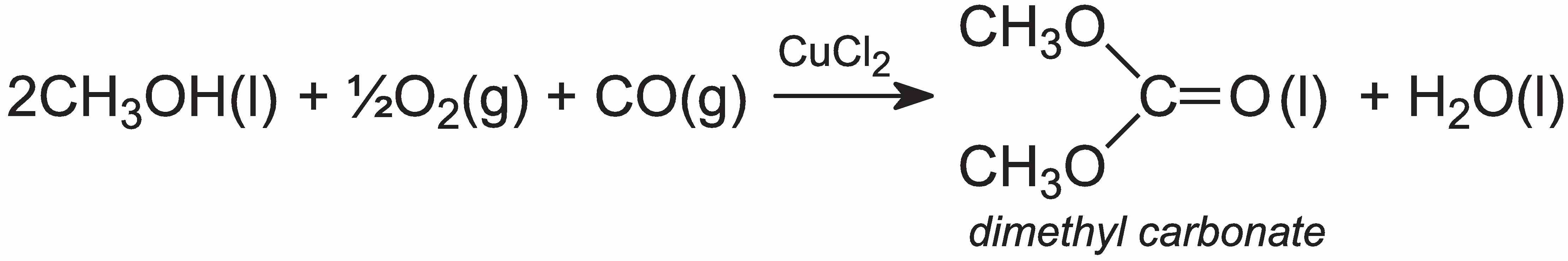
Dimethyl carbonate, when heated with phenol in the liquid phase, forms the diphenyl carbonate:
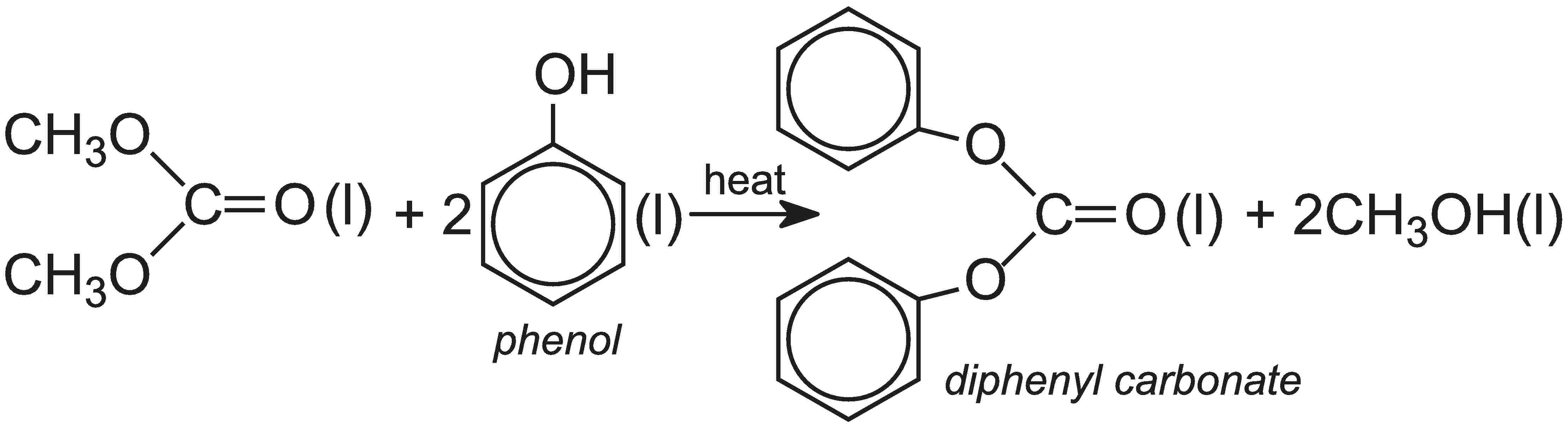
Overall, the process for the production of polycarbonate that uses diphenyl carbonate is less hazardous than that using carbonyl chloride.
Designing safer chemicals
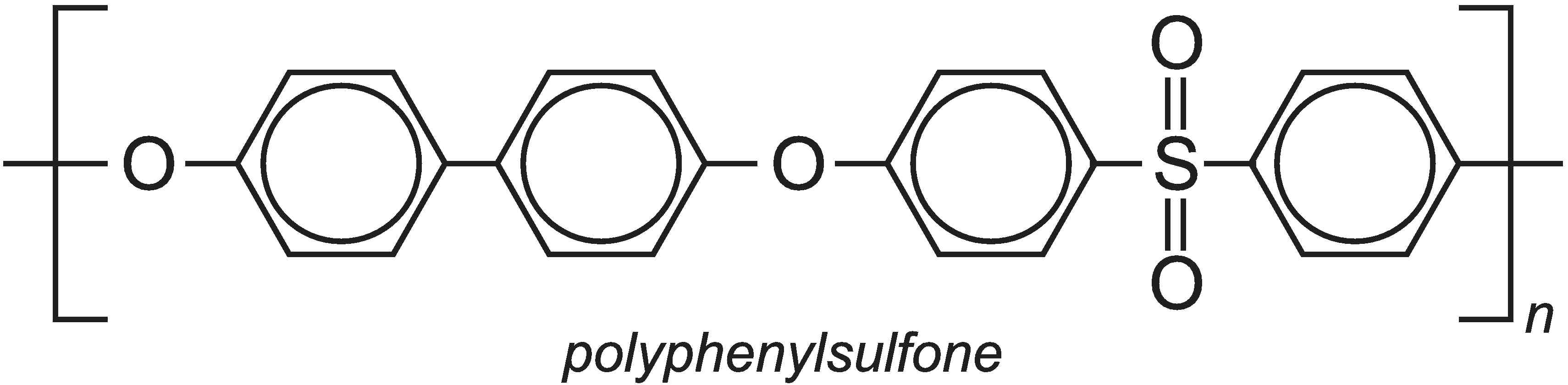
Underlying the concept of green chemistry is the desire to produce chemicals that are as useful as possible whilst also being both safe for us to use and safe for the environment. Safety refers to both flammability and toxicity. For example, polymers have been developed which are much less flammable than the more well known polymers but also retain properties such as toughness. They must be able to absorb severe impacts without cracking and breaking. One such polymer is polyphenylsulfone which has the formula:
|
||||||
It is also important that chemicals that are produced are safe for the environment. Some products are specifically intended to be spread on soil, used in water, sprayed in the air or ingested by people; others, like washing detergents, may end up in water courses or in household waste for landfill. In both these cases, the material should degrade to harmless products. Detergents used to be based on the sodium salts of alkylbenzene sulfonic acids, and the alkyl group was branched. These were not degraded naturally in sewage works and caused foaming which made the sewage difficult to manage. Now these compounds have been replaced with sodium salts of linear alkylbenzene sulfonic acids, which are readily degraded. Their production is not simple and it took much research to develop it.
Another development to help the environment was the replacement of the compounds added to detergents to remove magnesium and calcium ions from hard water, known as builders. Sodium phosphates were used for this purpose but these caused considerable problems leading to eutrophication of water courses. Now zeolites (aluminosilicates) are used which are benign.
Further examples are the pyrethoid pesticides which have the duel benefits of breaking down in sunlight in 2-3 days and have much lower acute toxicity to humans than phosphorus, or chlorine-based pesticides.
Safer solvents
Reactions that occur in the gas phase are preferable as they avoid the use of solvents to bring the reactants together. Examples include the manufacture of ammonia, the manufacture of methanol and the manufacture of ethene.
Some reactions use water as a solvent, for example in the manufacture of inorganic compounds such as hydrogen peroxide, phosphoric acid, sodium carbonate, and organic compounds such as ethane-1,2-diol and ethanol. Water is not a harmful solvent but it is a precious resource and it is important to ensure that it is not wasted.
In the manufacture of ethanoic acid, the product itself is used as the solvent. However, other reactions use organic solvents which readily evaporate into the atmosphere unless great care is used to contain them. Wherever possible alternative solvents are used which are not harmful, one example being the development of water-borne paints, which are replacing paints that use volatile organic compounds such as the hydrocarbons which are harmful to the atmosphere. Supercritical (liquid) carbon dioxide is widely used as a solvent in the extraction of caffeine from coffee beans and in the latest drycleaning equipment it replaces chlorinated solvents such as perchloroethene, C2Cl4.
Energy efficiency and use of waste materials
All manufacturing processes need energy to convert raw materials into useful products. In the chemical industry it is used to heat reactants and in processes such as distillation, product drying, electrolysis, and treatment of waste.
At present, the energy used still relies mainly on fossil fuels, but even so the use of these can be reduced in several ways (Table 2).
| Maintenance and recovery | Good insulation and well-maintained equipment will reduce heat loss, and any waste heat can be used for warming offices and producing hot water rather than being lost to the atmosphere. In some cases this heat may be shared with a local community by piping hot water from the site. |
| Reaction choice and conditions | Reactions and catalysts that operate at lower temperatures may be chosen. Most reactions based on biosynthesis work at relatively low temperatures; however this may need balancing with the extra energy often needed for product separation. |
| Combined heat and power (CHP) | Manufacturing sites often generate their own electricity, rather than buying from the grid. This is more efficient as it eliminates transmission losses, and the excess heat released during the generation process can be used on site for many different purposes from pre-heating reactants to keeping offices warm. |
Table 2 Improving energy efficiency in the chemical industry.
Waste often has energy content, and it may be possible to convert this to a useful fuel. Waste solvents from the manufacture of paints, varnishes, adhesives, inks, cleaning fluids and so on are made into a liquid fuel for use by the cement-making industry. A solid fuel is also made from shredded carpets, packaging, furniture, plastics and paper, most of which would otherwise be destined for landfill.
Old vehicle tyres can no longer be sent to landfill in the EU. Many are shredded and used as a fuel by the cement industry. A single plant may consume as many as 250 000 tyres annually.
All fuels of this type must meet strict criteria before use to prevent the production of harmful combustion products, and constant monitoring is essential to ensure the emissions remain within legal requirements.
Traditional processes are being overhauled and more energy efficient ones substituted. Catalysts are being developed so that a process can be run at lower temperatures and pressures (high temperatures and pressures are very energy consuming.
Similarly, the development of molecular sieves means that processes such as the purification of ethanol can be carried out at ambient temperatures instead of by distillation.
Some industries co-operate to make better use of energy. For example, the production of ammonia generates both waste heat and carbon dioxide, both derived from fossil fuel. One UK manufacturer pipes these to large commercial tomato greenhouses, greatly extending the season during which the plants may be grown economically. As well as saving fuel for heating greenhouses, fewer tomatoes need to be imported (saving 'air miles') and the time between picking and purchase is shorter, giving consumers fresher produce.
This use of waste carbon dioxide has recently been enhanced in Icelend in a particularly exciting development. The country is one of the pioneers in building power stations based on geothermal power. In geothermal power stations super-heated steam generated deep underground when water comes into contact with heated rock or magma from the earth’s mantle is extracted through a series of boreholes and piped into a turbine, where the steam is used to generate electricity.
Small amounts of carbon dioxide and other gases such as hydrogen sulphide are emitted from the geothermal areas. In one area in Iceland, the gases from a power plant are piped to an adjacent installation where carbon dioxide is separated from other non-condensable gases and used as an input to a process, where hydrogen and carbon dioxide are passed over a solid catalyst under high pressure to produce renewable methanol. The hydrogen is made by electrolysis of water using electricity from hydro and geothermal power sources. This green methanol can be blended directly with standard petrol or can be used in esterification of vegetable oil or animal fats to produce biodiesel (Fatty Acid Methyl Ester).
 |
| Figure 6 This plant in Iceland is capable of producing 1300 tonnes of methanol a year from waste carbon dioxide from geothermal sources. Its capacity will shortly increase to 4000 tonnes a year and the next generation will increase that tenfold. The technology is such that plants could be built adjacent to other industrial emission sources such as in the manufacture of cement and steel. By kind permission of Carbon Recycling International |
Renewable feedstocks
There are many developments aiming to reduce the dependence of the chemical industry on oil. These are discussed in detail in units devoted to biotechnology, biofuels and biorefineries.
Renewable resources are theoretically inexhaustible, and the range of materials being manufactured from such sources continues to grow. Examples on this website describe the production of a variety of compounds including the production of:
- surfactants are made which are readily biodegradable, and in some cases are manufactured from renewable plant-derived resources such as carbohydrates (sucrose, glucose) or plant oils.
- polyols, from soya, which are used to make polyurethanes.
- ethene from bioethanol, and which is used to make bio-based poly(ethene).
- propene is being produced by a variety of ways from materials produced in turn from biodegradable resources, The propene is used to manufacture bio-based poly(propene).
- 1,4-dimethylbenzene (para-xylene), from bio-based ethene, can be used to make polyesters.
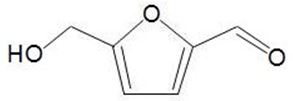
- a wide range of chemicals can be produced in chemocatalytic (bioforming) reactions, for example, hydroxymethylfurfural, HMF:
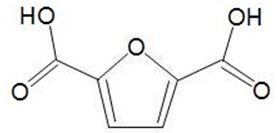
This can be oxidised to a dicarboxylic acid,
which can be used in place of benzene-1,4-dicarboxylic acid (terephthalic acid) and co-polymerised with a diol to make a polyester with similar properties to polyethylene terephthalate (PET).
Catalysis
Catalysts have played a huge part in the development of more sustainable processes for the manufacture of chemicals. There are many advantages in developing and using catalysts for industrial reactions, some important ones being:
- they affect the conditions that are needed, often reducing energy demand by lowering the temperature and pressure used
- they enable alternative reactions to be used which have better atom economy and thus reduce waste
- it is possible to control reaction pathways more precisely, reducing unwanted side products and making it easier to separate and purify the required product.
Attention is drawn in the twelve principles (Table 1) to the benefits of catalysts compared to stoichiometric reagents which are necessary for the reaction to take place but which cannot be recovered. For example, aluminium chloride was used for many years in the production of alkylbenzene sulfonates, an active surfactant in many detergents. The aluminium chloride was needed to effect the reaction between benzene and a long chain alkene. The aluminium chloride could not be recycled and became waste as aluminium hydroxide and oxide. Now a solid zeolite catalyst with acid groups is used and can be reused time and time again with no waste products.
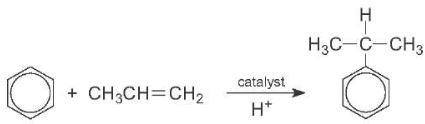
Similarly, benzene and propene are converted into cumene in the manufacture of phenol. This reaction needs an acid catalyst, such as aluminium chloride. A solid zeolite with acid groups, such as ZMS-5 is now the favoured catalyst:
The zeolite is more evironmentally friendly as the effluent is much cleaner and lower temperatures and pressures can be used.
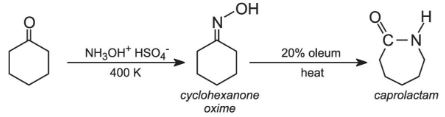
Another similar example is in the manufacture of one of the most important polymers used to make fabrics, polyamide 6 (sometimes known as nylon 6). In this process cyclohexanone is converted into caprolactam via the oxime (produced by the reaction of the ketone with hydroxylamine hydrogensulfate). The oxime is isomerised by sulfuric acid to caprolactam, the released sulfuric acid is converted to ammonium sulfate.
However, again a zeolite catalyst, with acidic sites, is now being used to effect the rearrangement. The zeolite is regenerated and saves the use and subsequent waste of sulfuric acid.
Another example is the removal of chlorine from effluents in sewage, which is usually present as the chlorate(I) (hypochlorite) ion. The ions are present because chlorination remains the most common form of waste water disinfection. However, this can lead to chlorination of residual organic material in the sewage, leading to chlorinated-organic compounds, which may be carcinogenic or harmful to the aquatic species.
One way of doing this is to reduce the hypochlorite ion to a chloride ion by adding solutions of nickel, iron or cobalt ions to the waste stream in stirred or agitated tanks. Another is to react the sewage with sulfur dioxide or a salt that will react with water to form it. Sulfur dioxide reduces the hypochlorite ion to chloride. However, it is not easy to handle and any escape can be very harmful.
A new process, known as HYDECAT (the Hypochlorite Decomposition Catalysis) uses very finely divided nickel dispersed on an inert solid. These pellets are on beds through which the effluent passes.
There is large surface area of the metal exposed to the effluent and the nickel leads to the reduction of the hypochlorite ion to the chloride ion and oxygen gas. The overall reaction is:
During the reaction the hypochlorite ion is adsorbed onto the catalyst surface where it is broken down to give a chloride ion with the oxygen atom, remaining on the catalyst surface, combining with an adjacent oxygen atom to form an oxygen molecule. The adsorbed oxygen atom can also oxidise harmful chlorinated-organic compounds. The catalyst can be readily regenerated.
The Hydecat process was originally designed to remove hypochlorite byproduct from waste streams generated during chlorination processes where sodium hydroxide scrubbers are used to remove excess chlorine, for example in the production of chloroethene (vinyl chloride), titanium dioxide (by the chloride route) and chlorofluorocarbons.
Design for degradation
Among the best known materials that are being produced intentionally for a limited life are degradable plastics. The new generation of surfactants, the alkylbenzene sulfonates have also been designed for rapid degradation.
Inherently safer chemistry for accident prevention
The impact of chemicals on human health and the environment can be the result of:
- routine or accidental emissions during production
- the use and disposal of a product.
It is not in the interest of any industry to waste resources or endanger its workforce, and this is as much an incentive to reduce emissions as are the legal requirements placed upon manufacturers. Some processes of necessity need the handling of dangerous materials but wherever possible industry is attempting to make this safer. One way is to alter the reagents used. For example, one process used in the manufacture of the most widely used herbicide, glyphosate (sold as Roundup), uses the sodium salt of 2,2'-iminodiethanoic acid as one of the intermediates. This is made in a series of reactions from ammonia, methanal (formaldehyde) and hydrogen cyanide. Although hydrogen cyanide is a very useful reagent, it is extremely toxic. A recent innovation has been the introduction of a new route to the sodium salt.
The starting materials are ammonia and epoxyethane, which, on reacting, form 2,2'-iminodiethanol, often named diethanolamine. This is then converted to the sodium salt of 2,2'-iminodiethanoic acid:
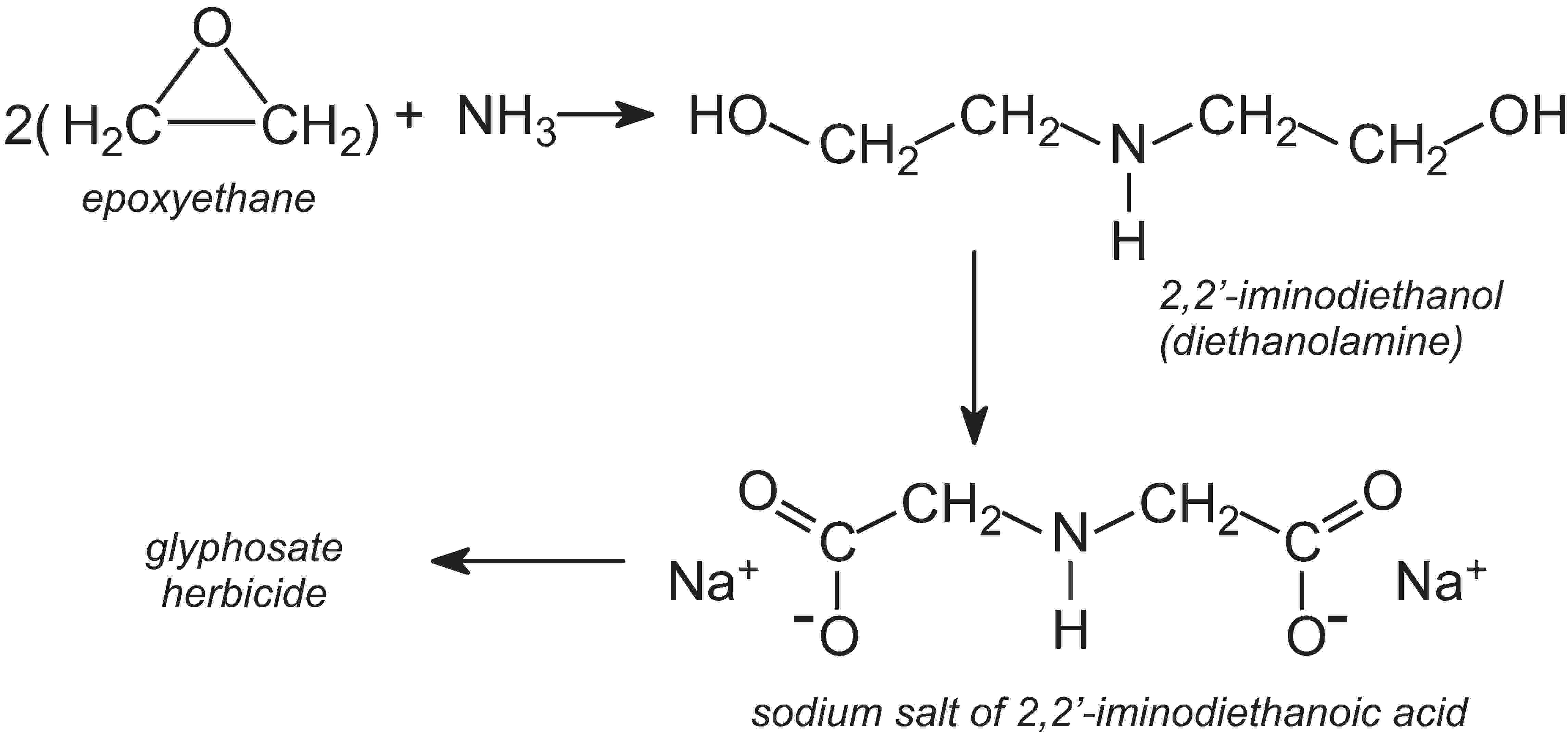
Thus in the event of an accident, the consequences would not be as serious, and clean-up would be simpler.
A manufacturing site will also generate waste in the form of unwanted material from the production processes; this may include solvents for reaction, extraction, purification and waste treatment. Solvents can often be recycled or, where this is not feasible, may be used as fuel substitutes (see above). Other waste may ultimately end up in landfill, and this is when the nature of the waste is important.
Many products are disposed of when they reach the end of their useful life. The ideal would be for all such waste to be recycled, rather than it ending up in landfill, though this is more dependent on the willingness of consumers to take responsibility. Products that are likely to go to landfill should be designed so they degrade rapidly and safely.
Cradle to grave
This unit has concentrated mostly on the changes that are being made to industrial production processes to make them more consonant with the principles of green chemistry. However a consideration of the environmental impact of a product from the time it is made until it is no longer required also requires a more detailed account of recycling and the degradation of disposed material (particularly plastics).
Date last amended: 30th October 2018