Catalysts are substances that speed up reactions by providing an alternative pathway for the breaking and making of bonds. Key to this alternative pathway is a lower activation energy than that required for the uncatalysed reaction.
Catalysts are often specific for one particular reaction and this is particularly so for enzymes which catalyse biological reactions, for example in the fermentation of carbohydrates to produce biofuels.
Much fundamental and applied research is done by industrial companies and university research laboratories to find out how catalysts work and to improve their effectiveness. If catalytic activity can be improved, it may be possible to lower the temperature and/or the pressure at which the process operates and thus save fuel which is one of the major costs in a large-scale chemical process. Further, it may be possible to reduce the amount of reactants that are wasted forming unwanted by-products.
If the catalyst is in the same phase as the reactants, it is referred to as a homogeneous catalyst. A heterogeneous catalyst on the other hand is in a different phase to the reactants and products, and is often favoured in industry, being easily separated from the products, although it is often less specific and allows side reactions to occur.
Heterogeneous catalysis
The most common examples of heterogeneous catalysis in industry involve the reactions of gases being passed over the surface of a solid, often a metal, a metal oxide or a zeolite (Table 1).
| Process | Catalyst | Equation |
|---|---|---|
| Making ammonia | Iron |  |
| Making synthesis gas (carbon monoxide and hydrogen) | Nickel |  |
| Catalytic cracking of gas oil | Zeolite | Produces: a gas (e.g. ethene, propene) a liquid (e.g.petrol) a residue (e.g. fuel oil) |
| Reforming of naphtha | Platinum and rhenium on alumina | 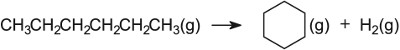 |
| Making epoxyethane | Silver on alumina | 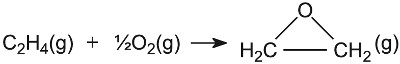 |
| Making sulfuric acid | Vanadium(V) oxide on silica | 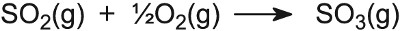 |
| Making nitric acid | Platinum and rhodium |  |
Table 1 Examples of industrial processes using heterogeneous catalysis.
The gas molecules interact with atoms or ions on the surface of the solid. The first process usually involves the formation of very weak intermolecular bonds, a process known as physisorption, followed by chemical bonds being formed, a process known as chemisorption.
Physisorption can be likened to a physical process such as liquefaction. Indeed the enthalpy changes that occur in physisorption are ca -20 to -50 kJ mol-1, similar to those of enthalpy changes when a gas condenses to form a liquid. The enthalpies of chemisorption are similar to the values found for enthalpies of reaction. They have a very wide range, just like the range for non-catalytic chemical reactions.
An example of the stepwise processes that occur in heterogeneous catalysis is the oxidation of carbon monoxide to carbon dioxide over palladium. This is a very important process in everyday life. Motor vehicles are fitted with catalytic converters. These consist of a metal casing in which there are two metals, palladium and rhodium, dispersed very finely on the surface of a ceramic support that resembles a honeycomb of holes. The converter is placed between the engine and the outlet of the exhaust pipe.
The exhaust gases contain carbon monoxide and unburned hydrocarbons that react with the excess oxygen to form carbon dioxide and water vapour, the reaction being catalysed principally by the palladium:
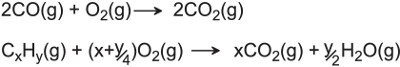
The exhaust gases also contain nitrogen(II) oxide (nitric oxide, NO), and this is removed by reactions catalysed principally by the rhodium:

The accepted mechanism for the oxidation of carbon monoxide to carbon dioxide involves the chemisorption of both carbon monoxide molecules and oxygen molecules on the surface of the metals. The adsorbed oxygen molecules dissociate into separate atoms of oxygen. Each of these oxygen atoms can combine with a chemisorbed carbon monoxide molecule to form a carbon dioxide molecule. The carbon dioxide molecules are then desorbed from the surface of the catalyst. A representation of these steps is shown in Figure 1.
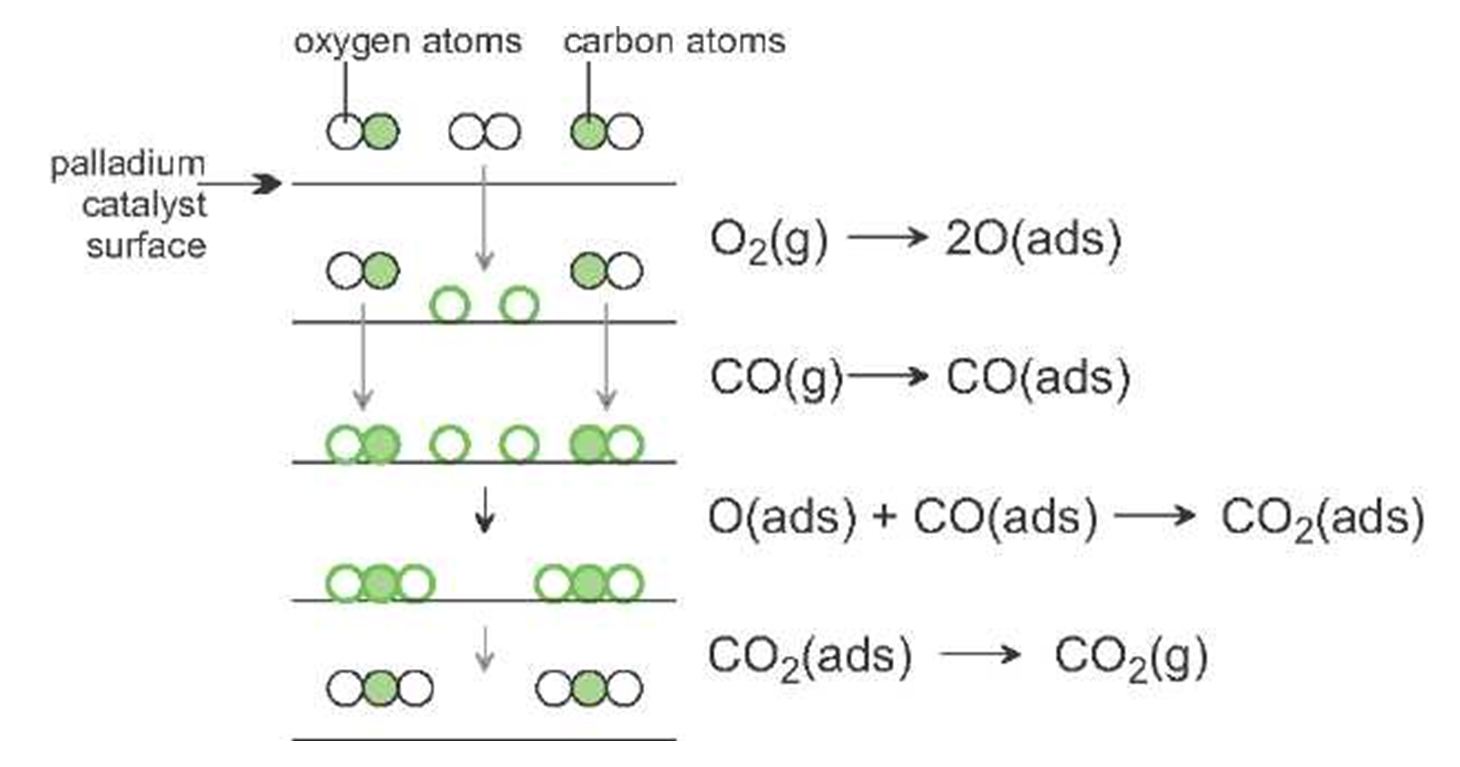
Figure 1 A mechanism for the oxidation of carbon monoxide.
Each of these steps has a much lower activation energy than the homogeneous reaction between the carbon monoxide and oxygen.
The removal of carbon monoxide, unburned hydrocarbons and nitrogen(II) oxide from car and lorry exhausts is very important for this mixture leads to photochemical smogs which aggravate respiratory diseases such as asthma.
Platinum, palladium and rhodium are all used but are very expensive metals and indeed each is more expensive than gold. Recently, much work has been devoted to making catalysts with very tiny particles of the metals, an example of the advances being made by nanotechnology.
It is not simply the ability of the heterogeneous catalyst's surface to interact with the reactant molecules, chemisorption, that makes it a good catalyst. If the adsorption is too exothermic, i.e. the enthalpy of chemisorption is too high, further reaction is likely to be too endothermic to proceed. The enthalpy of chemisorption has to be sufficiently exothermic for chemisorption to take place, but not so high that it does not allow further reaction to proceed. For example, in the oxidation of carbon monoxide, molybdenum might at first sight be favoured as a choice, as oxygen is readily chemisorbed by the metal. However, the resulting oxygen atoms do not react further as they are too strongly adsorbed on the surface. Platinum and palladium, on the other hand, have lower enthalpies of chemisorption with oxygen, and the oxygen atoms can then react further with adsorbed carbon monoxide.
Another point to consider in choosing a catalyst is that the product must not be able to adsorb too strongly to its surface. Carbon dioxide does not adsorb strongly on platinum and palladium and so it is rapidly desorbed into the gas phase.
A testimony to the importance of catalysis today is the award of the Nobel Prize in Chemistry in 2007 to Gerhard Ertl for his work in elucidating, amongst other processes, the mechanism for the synthesis of ammonia (the Haber Process):
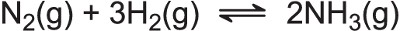
Ertl obtained crucial evidence on how iron catalyses the dissociation of the nitrogen molecules and hydrogen molecules leading to the formation of ammonia
(Figure 2):
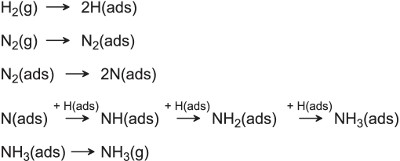
Figure 2 A mechanism for the catalytic synthesis of ammonia.
Figure 3 shows how the activation energy barriers are much lower than the estimated activation energy barrier (which would be at least 200 kJ mol1) for the uncatalysed synthesis of ammonia.
Figure 3 The activation energy barriers for the reactions occurring during the catalytic synthesis of ammonia.
General requirements for a heterogeneous catalyst
To be successful the catalyst must allow the reaction to proceed at a suitable rate under conditions that are economically desirable, at as low a temperature and pressure as possible. It must also be long lasting. Some reactions lead to undesirable side products. For example in the cracking of gas oil, carbon is formed which is deposited on the surface of the catalyst, a zeolite, and leads to a rapid deterioration of its effectiveness. Many catalysts are prone to poisoning which occurs when an impurity attaches itself to the surface of the catalyst and prevents adsorption of the reactants. Minute traces of such a substance can ruin the process, One example is sulfur dioxide, which poisons the surface of platinum and palladium. Thus all traces of sulfur compounds must be removed from the petrol used in cars fitted with catalytic converters.
Further, solid catalysts are much more effective if they are finely divided as this increases the surface area.
Figures 4 and 5 Two ways by which the surface area of a catalyst can be increased.
| In Figure 4, the platinum-rhodium alloy (used in the manufacture of nitric acid) is in the form of very fine wire that has been woven to construct a gauze. By kind permission of Johnson Matthey. In Figure 5, vanadium(V) oxide (used in the manufacture of sulfuric acid) has been produced in a 'daisy' shape. By kind permission of Haldor Topsøe A/S.  |
 |
At high temperatures, the particles of a finely divided catalyst tend to fuse together and the powder may 'cake', a process known as sintering. This reduces the activity of the catalyst and steps must be taken to avoid this. One way is to add another substance, known as a promoter. When iron is used as the catalyst in the Haber Process, aluminium oxide is added and acts as a barrier to the fusion of the metal particles. A second promoter is added, potassium oxide, that appears to cause the nitrogen atoms to be chemisorbed more readily, thus accelerating the slowest step in the reaction scheme.
| Figure 6 A platinum-rhodium gauze is used as a catalyst in the reaction between ammonia and methane to produce hydrogen cyanide, an intermediate in the production of methyl 2-methylpropenoate. The gauze operates at 1270 K and is thus glowing. The photograph was taken though a sight glass located on the reactor. | 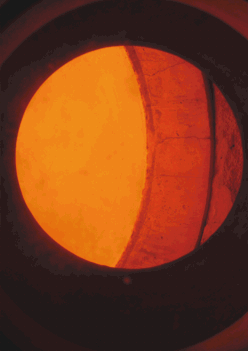 |
Aluminium oxide, silicon dioxide, aluminosilicates and zeolites
One of the most important reactions in which aluminium oxide, Al2O3, (often referred to as alumina) takes part in an industrial reaction is in platforming, in which naphtha is reformed over aluminina impregnated with platinum or rhenium. Both the oxide and the metals have catalytic roles to play, an example of bifunctional catalysis. There are hydroxyl groups on the surface of alumina which are, in effect, sites which are negatively charged to which a hydrogen ion is attached that can act as an acid catalyst.
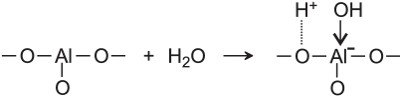
Silicon dioxide (silica) is another acidic oxide used in industry. It becomes particularly active if it has been coated with an acid (such as phosphoric acid), thereby increasing the number of active acidic sites. For example, the manufacture of ethanol is achieved by the hydration of ethene using silica, coated with phosphoric acid:
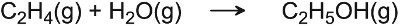
The mechanism involves the formation of a carbocation (Figure 7):
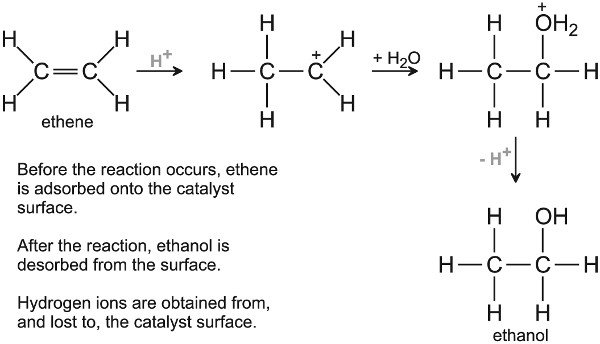
Figure 7 A mechanism for the hydration of ethene to ethanol.
Aluminosilicates are also used as catalysts when an acid site is required. These are made from silicon dioxide (silica) and aluminium oxide. They contain silicate ions, SiO44- that have a tetrahedral structure which can be linked together in several ways. When some of the Si atoms are replaced with Al atoms, the result is an aluminosilicate. Hydrogen ions are again associated with the aluminium atoms:

Zeolite catalysts
A particular class of aluminosilicates that has excited huge interest in recent years is the zeolites. There are many different zeolites because of the different ways in which the atoms can be arranged. Their structure of silicate and aluminate ions can have large vacant spaces in three dimensional structures that give room for cations such as sodium and calcium and molecules such as water. The spaces are interconnected and form long channels and pores which are of different sizes in different zeolites.
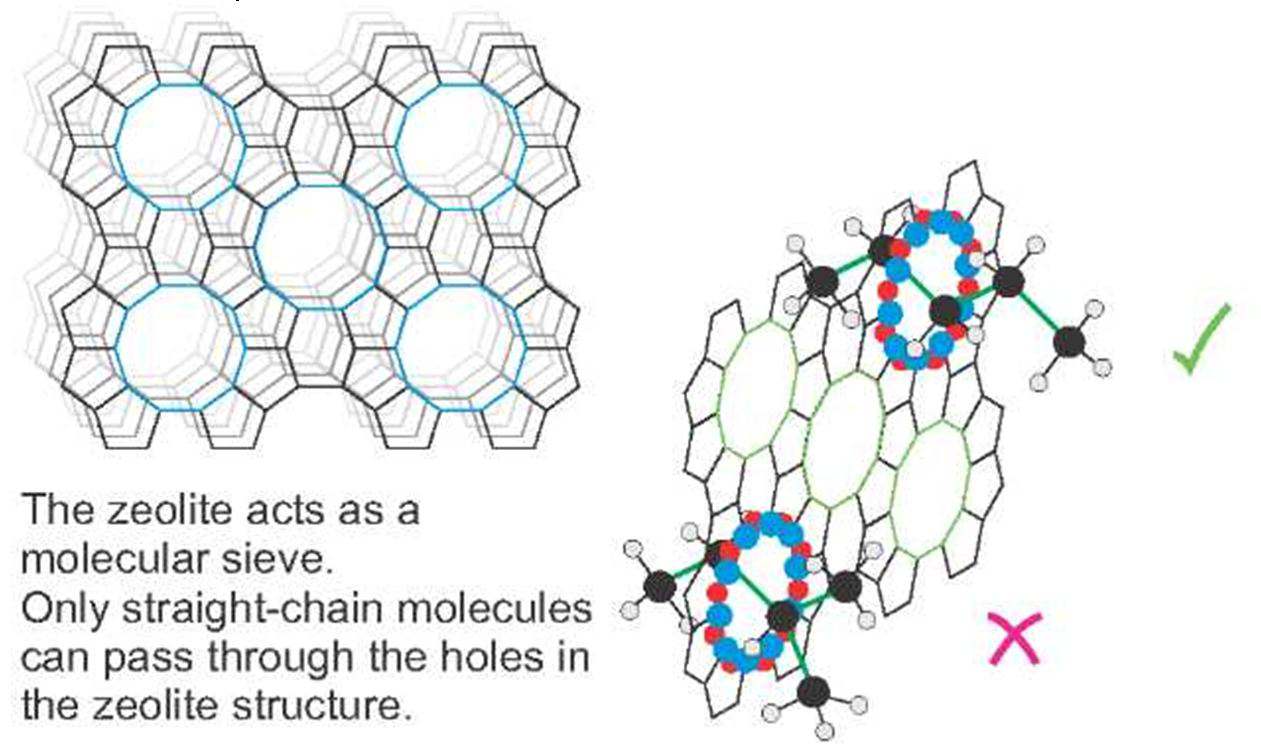
Figure 8 The structure of a zeolite (example figure)
A zeolite which is commonly used in many catalytic reactions is ZSM-5 which is prepared from sodium aluminate (a solution of aluminium oxide in aqueous sodium hydroxide) and a colloidal solution of silica, sodium hydroxide, sulfuric acid and tetrapropylammonium bromide.
It is, for example, a very effective catalyst for the conversion of methylbenzene (toluene) to the three dimethylbenzenes (xylenes). Alas, the mixture produced only contains about 25% 1,4-dimethylbenzene, (p-xylene) the isomer needed for the manufacture of the polyesters and the rest, 1,2- (o-xylene) and 1,3-dimethylbenzenes (m-xylene), is not wanted in such large quantities.
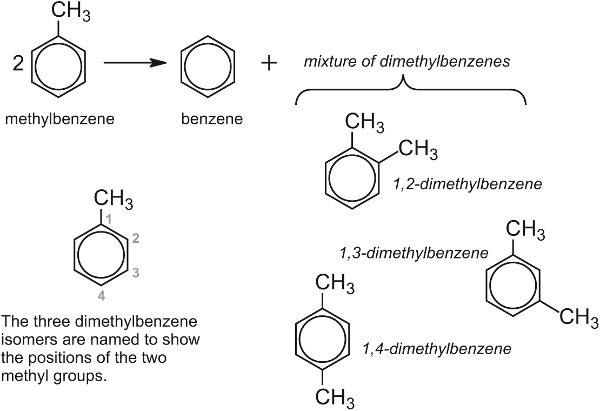
However, if the zeolite is washed with phosphoric acid and heated strongly, minute particles of phosphorus(V) oxide are deposited on the surface making the pores slightly smaller. This restricts the diffusion of the 1,2- and 1,3-isomers and they are held in the pores until they are converted into the 1,4-isomer and can escape (Figure 9).
This remarkable selectivity enables the yield of the 1,4-isomer to be increased from 25% to 97%.

| Figure 9 A zeolite acting an a molecular sieve and a catalyst during the formation of 1,4-dimethylbenzene from methylbenzene. |
The ability of the zeolite to adsorb some molecules and to reject others gives it the ability to act as a molecular sieve. For example, in the manufacture of ethanol from ethene or from biomass, an aqueous solution of ethanol is produced, in which there is 4% water still present even after repeated distillations. Further purification of ethanol requires the use of a zeolite which absorbs the water preferentially. Table 2 gives examples of industrial processes involving zeolites.
| Process | Catalyst | Equation |
|---|---|---|
| Catalytic cracking of gas oil | Zeolite |
Produces: a gas (e.g. ethene, propene) a liquid (e.g.petrol) a residue (e.g. fuel oil) |
| Reforming of naphtha
|
Platinum and rhenium on zeolite | 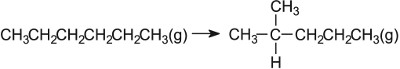 |
| Disproportionation of methylbenzene | Zeolite | 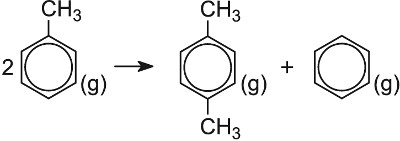 |
| Dealkylation of methylbenzene | Zeolites | 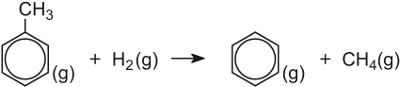 |
| Making cumene (1-methylethyl)benzene< | Zeolite (ZSM-5) | 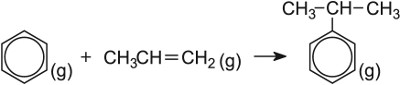 |
Table 2 Examples of industrial processes using zeolites.
Bifunctional catalysts
Bifunctional catalysts are able, as the name implies, to catalyse the conversion of one compound to another, using two substances on the surface.
For example, in reforming naphtha (a mixture of straight chain alkanes, with 6-10 carbon atoms) a bifunctional catalyst is used. The most well known one is platinum impregnated on the surface of alumina and both the metal and the oxide play their parts in the process. As can be seen (Figure 10), the first step is the dehydrogenation of the alkanes to alkenes, catalysed by the metal, followed eventually by adsorption of the alkene molecules on alumina. Because platinum is involved, the reforming is sometimes called platforming. The hydrogen ensures that the resulting alkenes and cycloalkenes subsequently react with hydrogen to form saturated compounds.
In this example butane is dehydrogenated to butene.
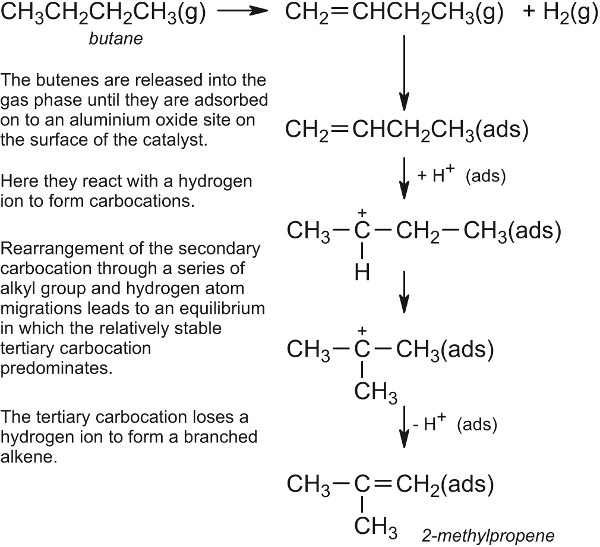
Figure 10 A mechanism for the reforming of butane to 2-methylpropene (isobutene).
The branched alkene molecule is desorbed into the gas phase until it is readsorbed on to a metal site where it is hydrogenated to form a branched alkane, 2-methylpropane (isobutane), which is then desorbed into the gas phase.
In the industrial process, naphtha vapour is passed over platinum and rhenium (ca 0.3% each) which are finely dispersed over aluminium oxide.
The rhenium is thought to play an interesting role. If a sulfur compound is allowed to pass over the surface of the catalyst, it is preferentially adsorbed by the rhenium. If sulfur compounds are not removed, reactions occur leading eventually to the formation of carbon which causes the activity of the catalyst to be markedly reduced.
Branched alkanes have a much higher octane rating than straight chain ones. Not only are the alkanes now branched, but cycloalkanes are also formed and, from them, aromatic hydrocarbons. All three classes of hydrocarbon have a higher octane rating than naphtha. Besides aluminium oxide and silicon dioxide, other oxides are important catalysts. For example, in the Contact Process used to manufacture sulfuric acid, the catalyst for the oxidation of sulfur dioxide to sulfur trioxide is vanadium(V) oxide on the surface of silica:
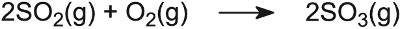
Potassium sulfate is added as a promoter. Its mode of action is not absolutely clear but it appears to be because its presence lowers the melting point of the catalyst, and allows it to spread out as a very thin layer over the entire surface.
Several important industrial processes are catalysed by mixed metal oxides. The surfaces contain two or more different metal atoms, O2- ions and -OH groups. They are particularly useful in the oxidation of hydrocarbons, where selective oxidation is required. For example, propene can be oxidized to propenal (acrolein) using a mixture of bismuth(III) and molybdenum(VI) oxides.
Without the catalyst, propene is oxidized to a large number of organic compounds, including methanal and ethanal, and eventually forming carbon dioxide. The oxygen atoms on the surface of molydenum(VI) oxide are not very reactive, reacting selectively with propene and breaking the weakest bond in the alkene to form an allyl radical:
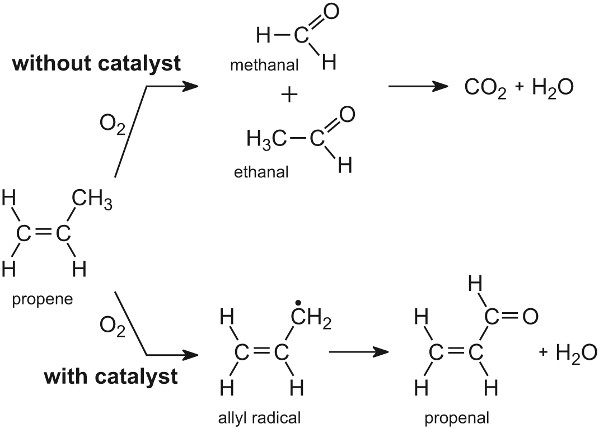
Figure 11 The oxidation of propene
The allyl radical is then oxidized on the surface to yield propenal. It is postulated that the allyl radical is oxidized by an oxygen atom that is adsorbed at a molybdenum site. Another oxygen atom, adsorbed on a bismuth site, is then transported to the reduced molybdenum site to replace that oxygen. There is a compensating transport of electrons to complete the cycle.
The same catalyst is also used to manufacture propenonitrile:
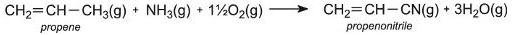
Homogeneous catalysis
Homogeneous catalysts are less frequently used in industry than heterogeneous catalysts as, on completion of the reaction, they have to be separated from the products, a process that can be very expensive.
| Manufacture | Catalyst | Equation |
|---|---|---|
| Ethane-1,2-diol | Sulfuric acid | 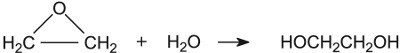 |
| 2,2,4-Trimethylpentane (iso-octane) |
Hydrogen fluoride | 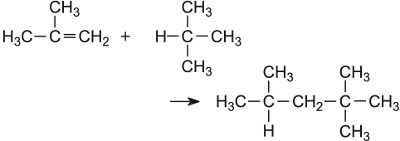 |
| Phenol and propanone | Sulfuric acid | 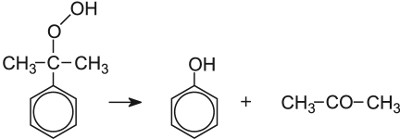 |
| Bisphenol A | Sulfuric acid | 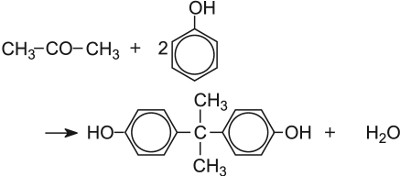 |
Table 3 Examples of industrial processes using homogeneous catalysis.
However, there are several important industrial processes that are catalysed homogeneously, often using an acid or base (Table 3).
One example is in the manufacture of ethane-1,2-diol from epoxyethane where the catalyst is a trace of acid:

Figure 12 A mechanism for the formation of ethane-1,2-diol from epoxyethane.
In the mechanism for this reaction a hydrogen ion is added at the start, and lost at the end. The hydrogen ion functions as a catalyst.
Two other examples are concerned with the production of 2,2,4-trimethylpentane from 2-methylpropene, again using an acid as the catalyst. One uses 2-methylpropane (Table 3) which yields the alkane directly. The other uses only 2-methylpropene.
The mechanism of the reaction also involves the addition of a hydrogen ion to a reactant (Figure 13).

Figure 13 Part of a mechanism for the formation of 2,4,4-trimethyl-2-pentene from 2-methylpropene.
The alkene is then hydrogenated, using nickel as the catalyst, to 2,2,4-trimethylpentane (isooctane):
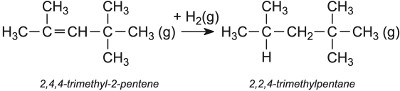
2,2,4-trimethylpentane is often added to petrol to enhance its anti-knock properties, now that methyl t-butyl ether (MTBE) is being phased out.
Catalysts for polymerization reactions
Ziegler-Natta catalysts
Ziegler-Natta catalysts are organometallic compounds which act as catalysts for the manufacture of poly(ethene) and poly(propene). For their work on the production of polyalkenes, Karl Ziegeler and Giulano Natta were awarded the Nobel Prize in Chemistry in 1965. The catalysts are prepared from titanium compounds with an aluminium trialkyl which acts as a promoter:
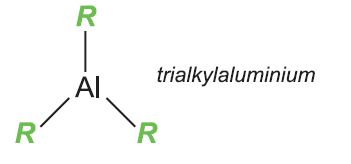
The alkyl groups used include ethyl, hexyl and octyl.
The role of the titanium catalyst can be represented as shown in Figure 14.
The alkene monomer, for example ethene or propene, attaches itself to an empty coordination site on the titanium atom and this alkene molecule then inserts itself into the carbon-titanium bond to extend the alkyl chain. This process then continues, thereby forming a linear polymer, poly(ethene) or poly(propene).
The polymer is precipitated when the catalyst is destroyed on addition of water. Because it is linear, the polymer molecules are able to pack together closely, giving the polymer a higher melting point and density than poly(ethene) produced by radical initiation.
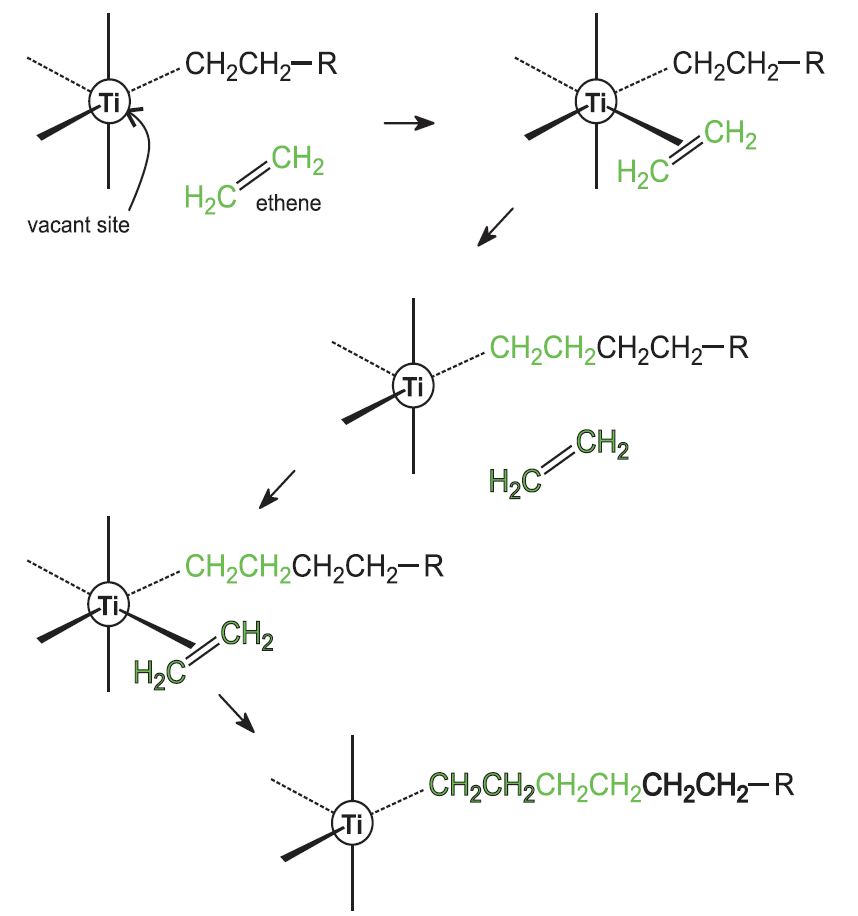
Figure 14 Illustrating the role of a Ziegler-Natta catalyst.
Not only do Ziegler-Natta catalysts allow for linear polymers to be produced but they can also give stereochemical control. Propene, for example could polymerize, even if linear, in three ways, to produce either atactic, isotactic or syndiotactic poly(propene).
However, this catalyst only allows the propene to be inserted in one way and isotactic polypropene is produced.
Even greater control of the polymerization is obtained using a new class of catalysts, the metallocenes.
Radical polymerization
Many polymers are produced using radical initiators, which act as catalysts (Table 4). For example the polymerization of chloroethene is started by warming it with a minute trace of a peroxide (R-O-O-R):
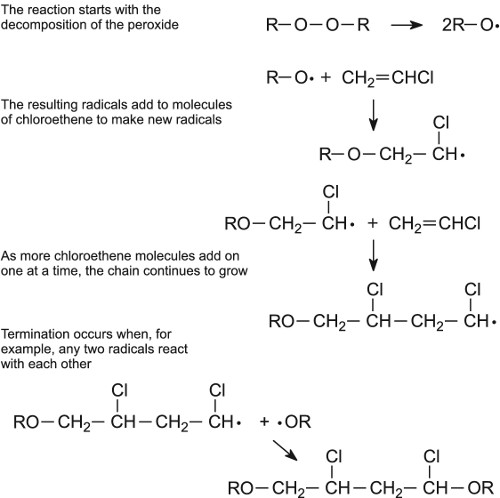
Figure 15 A mechanism for the free radical polymerization of chloroethene to poly(chloroethene).
In the case of ethene, by using the free radical process, the resulting polymer has a lower density and a lower softening point than the poly(ethene) produced using a Ziegler-Natta catalyst or a metallic oxide. The low density poly(ethene), LDPE, has side chains because the radicals react not only with molecules of ethene, by addition, but also with polymer molecules, by a process known as hydrogen abstraction. The polymer radical can also abstract a hydrogen atom from its own chain:
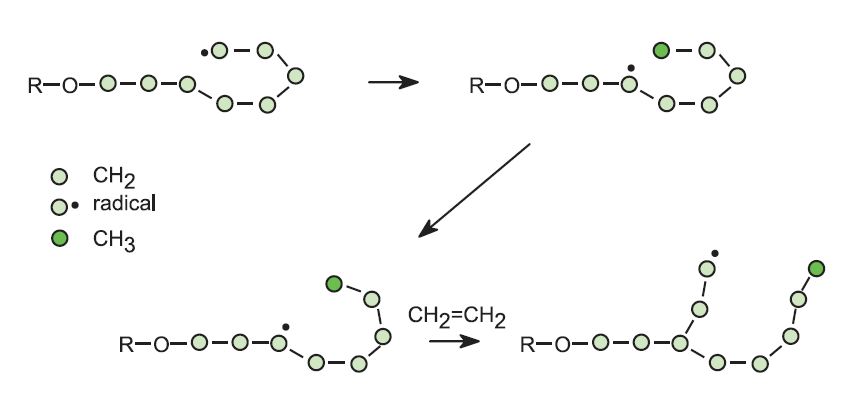
Both of these reactions lead to side chains so that the molecules of the polymer cannot pack together in a regular way. The polymer thus has a lower melting point and lower density.
| Monomer | Formula | Polymer | Structure |
|---|---|---|---|
| Ethene | 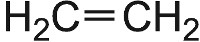 |
LDPE Poly(ethene) | 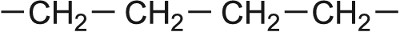 |
| Chloroethene | 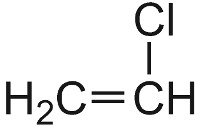 |
Poly(chloroethene) | 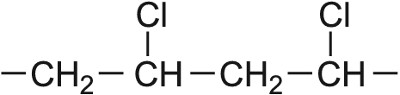 |
| Propenonitrile | 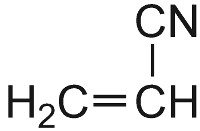 |
Poly(propenonitrile) | 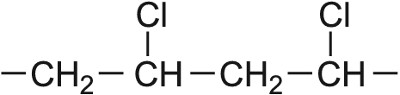 |
| Methyl 2-methylpropenoate | 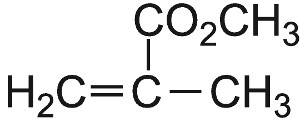 |
Poly(methyl 2-methylpropenoate) | 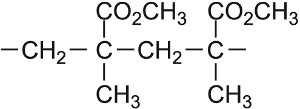 |
| Phenylethene | 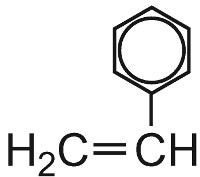 |
Poly(phenylethene) | 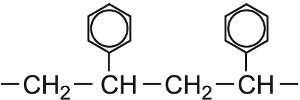 |
| Tetrafluorothene | 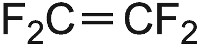 |
Poly(tetrafluoroethene) (PTFE) | 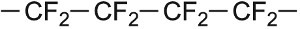 |
Table 4 Examples of polymers produced using free radical polymerization
Looking forward
The search for catalysts will continue to be one of the highest priorities for the chemical industry as it seeks to run the processes at as low a temperature and as near atmospheric pressure as possible, commensurate with a reasonable rate of reaction.
The gains from improving catalysts are both financial and environmental, leading to lower fuel costs, for example the manufacture of methanol and the reduction of harmful waste gases, for example the manufacture of ethanoic acid. Similarly, benzene and propene are converted into cumene in the manufacture of phenol, using a zeolite catalyst in place of aluminium chloride. This means lower temperatures and pressures are used and the effluent produced is much cleaner.
Further, catalysts are sought which will favour one specific reaction over another, thus again making the process much more economic. There are benefits if a catalyst can be used rather than another chemical that takes part stoichiometrically in the reaction and cannot be recovered and reused. For example, aluminium chloride was used for many years to effect the reaction between benzene and a long chain alkene in the production of alkylbenzene sulfonates, an active surfactant in many detergents. The aluminium chloride could not be recycled and became waste as aluminium hydroxide and oxide. Now a solid zeolite catalyst with acid groups is used and can be reused time and time again with no waste products.
Another similar example is in the manufacture of one of the most important polymers used to make fabrics, polyamide 6 (sometimes known as nylon 6). In this process, cyclohexanone is converted into caprolactam via the oxime (produced by the reaction of the ketone with hydroxylamine hydrogensulfate). The oxime is isomerised by sulfuric acid to caprolactam, and ammonium sulphate is produced as a by-product. However, again a zeolite catalyst, with acidic sites, is now being used to effect the rearrangement. The zeolite is regenerated and saves the use and subsequent waste of sulfuric acid.
Date last amended: 10th May 2013

