 Three types of calcium carbonate-containing rock are excavated and used by industry. They are limestone, chalk and dolomite. Limestone and chalk are both forms of calcium carbonate and dolomite is a mixture of calcium and magnesium carbonates. All have impurities such as clay but some rocks are over 97% pure. Limestone and other products derived from it are used extensively in the construction industry and to neutralise acidic compounds in a variety of contexts.
Three types of calcium carbonate-containing rock are excavated and used by industry. They are limestone, chalk and dolomite. Limestone and chalk are both forms of calcium carbonate and dolomite is a mixture of calcium and magnesium carbonates. All have impurities such as clay but some rocks are over 97% pure. Limestone and other products derived from it are used extensively in the construction industry and to neutralise acidic compounds in a variety of contexts.
In the chemical industry, large quantities of limestone are heated to ca 1500 K to form calcium oxide, known as quicklime:

Water can be added to lime to form calcium hydroxide. The process is known as 'slaking'. Solid calcium hydroxide is known as slaked lime or hydrated lime, and solutions and suspensions in water as milk of lime.
The term lime is often used to cover quicklime, slaked lime (hydrated lime) and milk of lime.
For a particular use, an appropriate choice is made from the four: limestone, quicklime, slaked lime or milk of lime. In many uses, lime reacts more quickly than limestone but is more expensive, because a high temperature is required to produce it from limestone.
Uses of limestone and lime
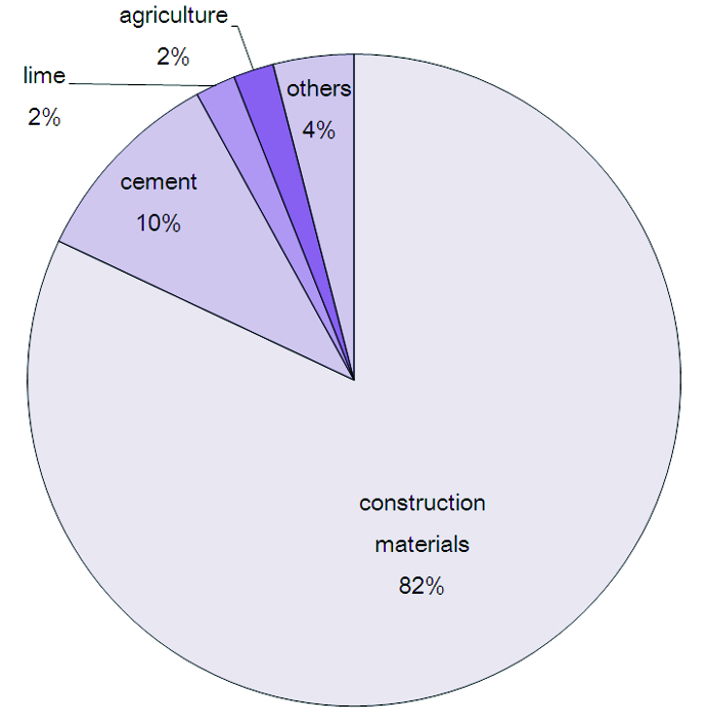
Figure 1 Principal uses of limestone and lime.
The principal uses, by far, of limestone and lime are in the construction industry and cement making. They are also used in the chemical and metallurgical industries and in agriculture.
On a worldwide basis, the proportions of lime used in different industries are:
- 60% metallurgy (mainly steel manufacture, slag formation and its use in the blast furnace)
- 25% construction (for example, it is used with asphalt in road paving, to stabilize soils and in making mortar and plaster)
- 15% for chemical and industrial uses (for example to make bleaches used in the manufacture of paper, to make precipitated calcium carbonate, a fine powder used in coatings for paper and paints, and in refining sugar to remove colloidal impurities) and for environmental uses (for example, with soda ash by both municipal authorities and industry, to soften water (remove calcium and magnesium ions), in the treatment of sewage to remove colloidal particles and in flue gas desulfurization)
However, these proportions vary widely from country to country. For example, in the US, the proportions are:
- 38% metallurgy (mainly steel manufacture)
- 31% environmental uses (for example, with soda ash, by both municiipal authorities and industry to soften water (remove calcium and magnesium ions), in the treatment of sewage, to remove colloidal particles and in flue gas desulfurization
- 22% chemical and industrial uses (for example to make bleaches used in the manufacture of paper, to make precipitated calcium carbonate, a fine powder used in coatings for paper and paints and in refining sugar to remove colloidal impurities)
- 8% construction uses (for example, it is used with asphalt in road paving, to stabilize soils and in making mortar and plaster)
- 1% others
Data from the US Geological Survey, 2012
The uses are described below, in more detail, in terms of different industries.
In the construction industry
Limestone has been used as a building material since the Stone Age. Indeed, the largest use of limestone and the various forms of lime is still in the construction industry, particularly in road building and building projects, from vast in size, bridges and skyscrapers, to houses. Large lumps of calcium carbonate are often used where sizeable quantities of aggregate are needed, for example for the foundations of roads.
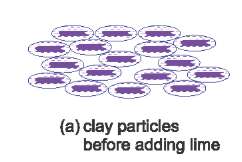
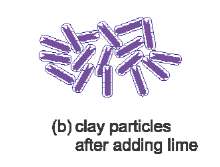
Lime is often used to make soil firmer. It reacts with clay minerals in the soil to form cement-like compounds (for example calcium silicate and calcium aluminate (calcium aluminosilicate)), Figure 2.
 |
 |
| Figure 2(a) Clay particles are surrounded by water, allowing them to be aligned and able to slide easily. This results in a clay soil with a low strength. |
Figure 2(b) When lime is added, the amount of water around the clay particles is reduced. The clay particles are no longer able to slide easily and the soil is strengthened. |
The strengthening of soil enables the construction of buildings by giving a more stable foundation. Lime is also used on building sites to allow large vehicles to move more easily (Figure 3).
|
Figure 3 The wet soil has been made harder by the addition of lime. This earth-moving equipment is able to move around easily. By kind permission of Singleton Birch. |
 |
Limestone is also the main constituent of cement and concrete.
In cement making
Cement is made by first mixing limestone and substances such as clays (which contain silica, alumina and iron(III) oxide) to a fine powder. The mixture is crushed and fed into a rotary kiln, which is an iron pipe, 60-90 m long and approximately 6 m in diameter. The pipe is rotated and heated to about 1700 K by flame inside of it. The kiln is slightly inclined to allow for the materials to slowly reach the other end, where it is quickly cooled by air to 373-473 K.
|
The heated air from the coolers is returned to the kilns, a process that saves fuel and increases burning efficiency.
The resulting solid comes out of the kiln as grey balls, about the size of marbles and is known as clinker. The clinker is ground to a fine powder and mixed with calcium sulfate (gypsum) to make the familiar grey fine powder. This is cement, it is essentially a mixture of calcium silicates and calcium sulfate. About 50% of cement (often known as Portland cement) is tri-calcium silicate, which hydrates and gives the cement its initial strength and about 25% is di-calcium silicate, which hydrates more slowly and gives added strength after a few days. When it is mixed with water, chemical reactions occur to form a hard solid, impervious to water. The role of the calcium sulfate is to prevent the cement from setting too quickly.
About 3.6 billion tonnes of cement are produced annually, of which China accounts for 2.0 billion tonnes and India 280 million tonnes1. Cement powder is usually mixed with sand and aggregate (gravel, granite) and when needed, they are mixed with water to form concrete.
1. U.S. Geological Survey, Mineral Commodity Summaries, 2016
|
Figures 5 and 6 Builders and masons, prior to the use of modern cement, used a mixture of lime (calcium hydroxide), sand and water, known as lime mortar or simply mortar. It has been used for over 6000 years, since the buildings of Ancient Greece and Rome. When these buildings are renovated, lime mortar is used rather than cement. In these photographs of York Minster, a relatively modern building dating back only 900 years, lime mortar has always been used in renovating the stonework that has to be replaced because of weathering over the centuries. By kind permission of Jessica Waddington. |
 |
 |
In industry and the environment
Many lakes have become too acidic because of aerial pollution (acid rain), for example in the US, Scandinavia and Scotland. The lakes are sprayed with very finely powdered calcium carbonate (Figure 7). Another effective way to treat this problem is to apply the powdered limestone to unplanted areas near to the sources of the streams leading to the lakes.

Figure 7 Powdered limestone is being sprayed over a lake near Hemsjö, in Southern Sweden.
By kind permission of Rickard Gillberg.
Limestone and the various forms of lime are used in large quantities to clean up the environment, by neutralising acids. For example, limestone and quicklime are used to remove sulfur dioxide produced in the burning of coal in power stations. Even 'clean' coal can contain about 1% sulfur.
The gaseous effluents, flue gases, from the burning of the coal are passed through a spray of very finely ground limestone or quicklime suspended in water. The sulfur dioxide, being an acid, reacts with them, for example:
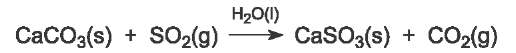
The resulting calcium sulfite collects at the base of the absorber and compressed air is blown into this residue. The calcium sulfite reacts with the air to form calcium sulfate (gypsum), used to make, for example, plaster board and cement.
Very fine and pure calcium carbonate is used as filler in plastics and paper. A filler is a substance that gives bulk but does not alter the properties of the substance to which it is added and is also inert. Calcium carbonate when very finely crushed (less than 2 microns) is used in paints to give a 'matt' finish.
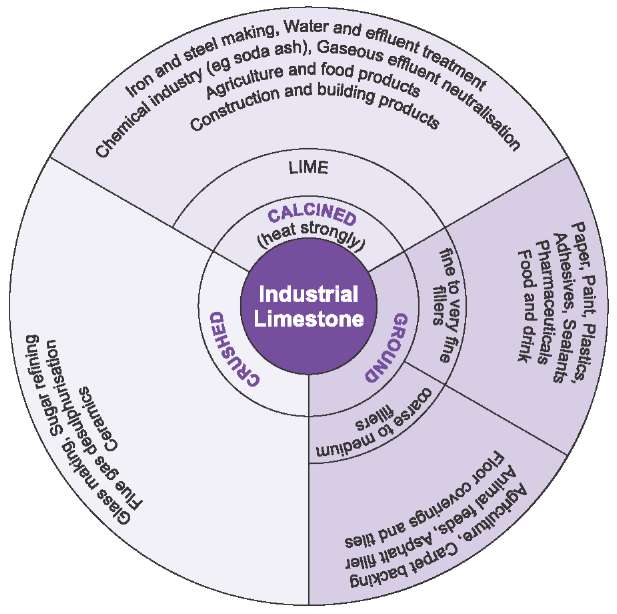
Figure 8 Uses of limestone.
Calcium carbonate is also used:
- to make sodium carbonate by the Solvay process
- in the blast furnace to make iron
- in the manufacture of glass
The uses are further summarized in Figure 8.
In agriculture
Crushed limestone and lime in all its forms are used to neutralize acids in the soil and so create the optimum soil conditions for crop growth. They also help to break down clays as described above, improving the soil structure, thus improving drainage and reducing soil erosion. Further, they provide a source of calcium ions that are an important plant nutrient.
Annual production of limestone
Data for the annual production of calcium carbonate are not readily available. Approximately 1 billion tonnes of its two principal ores, limestone and dolomite, are mined annually in the US. Given the relative amounts of lime that is used in different countries, an estimate of worldwide mining of calcium carbonate is 15 billion tonnes a year.
Annual production of lime (calcium oxide and calcium hydroxide)
| World | 350 million tonnes |
| China | 230 million tonnes |
| U.S. | 19 million tonnes |
| India | 16 million tonnes |
| Russia | 11 million tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016
Manufacture of calcium oxide (quicklime)
Calcium carbonate (limestone) is heated to form calcium oxide (quicklime) and carbon dioxide:
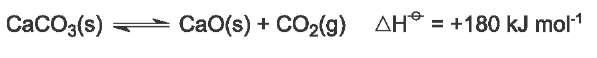
It is an endothermic reaction and the equilibrium lies far to the left at low temperatures. Only at about 1200 K does the partial pressure of carbon dioxide exceed atmospheric pressure and the decomposition proceeds to completion.
Quicklime is produced in refractory-lined kilns. Many designs are used, but the most common are based on the Vertical Shaft Kiln (Figure 9).
The kiln is made of steel, lined with refractory bricks. The limestone is fed in from the top and air is either sucked by fans or pushed by roots type blowers, through the kiln from the bottom (counter flow). The fuel is fed through the sides of the kiln, using about 8-10 lances inserted around the kiln.
The lime kiln consists of three principle zones:
- in the preheating zone, the heat in the combustion products, including carbon dioxide from the dissociation of limestone, is used to preheat the limestone to 1200 K
- in the burning zone the limestone is decomposed to quicklime at gas temperatures of 1500 K, a process known as calcining
- in the cooling zone, the heat in the quicklime leaving the burning zone is used to preheat the air required for combustion to 600-750 K.
The fuels used vary, depending on what is available. Many kilns use natural gas but in others oil is pumped in. In others, solids, such as finely powdered coal is pressurized and pumped in so that it acts as a fluid.
If there is not enough air to complete combustion of the fuel, more is fed in directly to the burning zone.
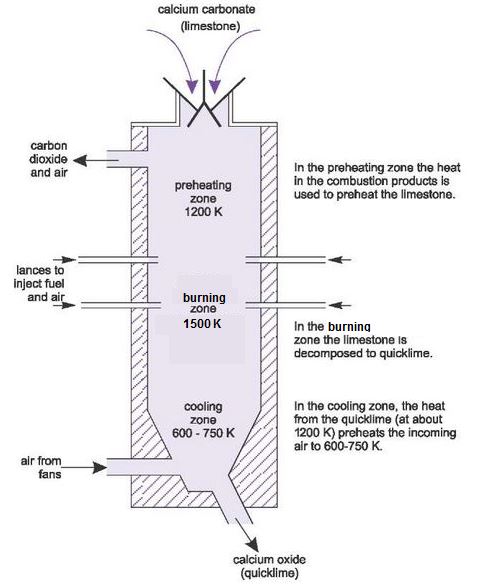
Figure 9 Manufacture of lime: The vertical shaft kiln.
Many recently introduced kilns have two parallel units (Figure 10). They are known as two-shaft kilns. In the simple single kiln, described above, the air is pumped up the kiln as the limestone flows down. This is known as a counter flow system. In the two-shaft kiln, known as the Parallel Flow Regenerative (PFR) lime kiln, the air and combustion gases travel parallel to the limestone. The fuel is injected just above the burning zone and the limestone absorbs most of the heat released by the fuel and so the temperature of the burning zone can be reduced to 1400 K, the temperature of the decomposition of limestone.
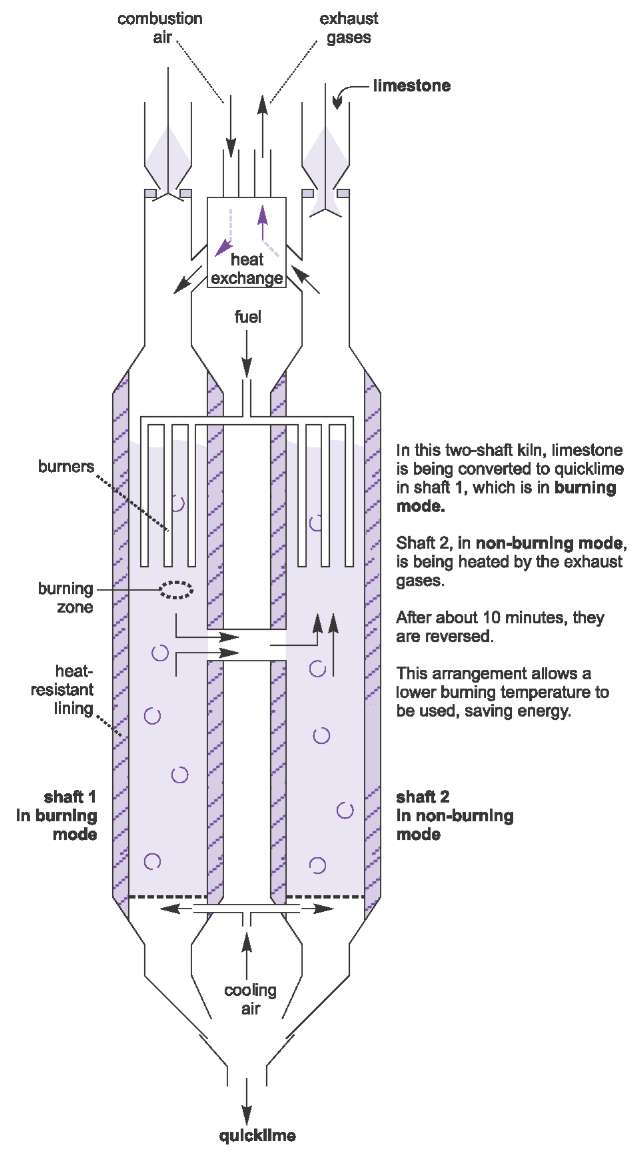 |
|
| Figure 10 Manufacture of lime: The Parallel Flow Regenerative (PFR) lime kiln. By kind permission of Maerz Ofenbau AG. |
Figure 11 A Parallel Flow Regenerative (PFR) lime kiln in Mexico. By kind permission of Maerz Ofenbau AG. |
In the diagram, the left-hand shaft is described as in burning mode. The exhaust gases cross over into the right-hand shaft, which is said to be in non-burning mode.
These hot gases travel up in counterflow to the stone, heating it up ready for the right-hand shaft to become the burning shaft and the left-hand shaft becomes non-burning.
Each shaft cycles through the burning and non-burning mode about every 10 minutes.
There is a large reduction in the fuel used by the two-shaft kiln compared to the single shaft kiln.
Shaft kilns have capacities for producing up to 800 tonnes of lime per day.
Quicklime is generally sold either as granules or as a finely ground powder.
Manufacture of calcium hydroxide (hydrated lime)
The reaction of quicklime with water is exothermic.
Powdered calcium hydroxide is produced by hydrating quicklime with a controlled excess of water to make a dry product. Milk of lime is prepared by slaking quicklime with an excess of water.
Date last amended: 16th January 2017


