.jpg) Butadiene is manufactured from fractions obtained from the distillation of oil. Although by far its major use is in the manufacture of artificial rubbers, some is used to make precursors in the manufacture of polyamides (nylon).
Butadiene is manufactured from fractions obtained from the distillation of oil. Although by far its major use is in the manufacture of artificial rubbers, some is used to make precursors in the manufacture of polyamides (nylon).
Uses of butadiene
By far the greatest use of butadiene is in the manufacture of co-polymers produced from phenylethene (styrene) and butadiene, SBS and propenonitrile (acrylonitrile), butadiene and phenylethene (styrene), ABS. The next biggest use is in the manufacture of poly(butadiene), a synthetic rubber, which is principally used as one of the components in the rubber used for car tyres:
.jpg)
Other synthetic rubbers made from butadiene include neoprene which is poly(2-chlorobuta-1,3-diene), often known as polychloroprene. Chloroprene is made from butadiene, by first reacting it with chlorine in the gas phase at ca 500 K to form 3,4-dichlorobut-1-ene and 1,4-dichlorobut-2-ene. The former, on reaction with sodium hydroxide, yields chloroprene:
.jpg)
On polymerization, neoprene is formed:
.jpg)
Neoprene, in solution, is an excellent adhesive and can also coat aluminium used in food packaging. It is widely used in cars, as hoses, vibration mounts and shock
absorber seals. It is also used widely in construction, for example in window seals and in large constructions such as bridges.
Another synthetic co-polymer made from butadiene is NBR (Nitrile Butadiene Rubber), the other monomer being propenonitrile (acrylonitrile). NBR's ability to withstand a range of temperatures from 220 to 380 K allows it be used in several important aeronautical applications, for example to make fuel and oil handling hoses and seals, where ordinary rubbers cannot be used. It is also used to make protective gloves for use in the nuclear industry. NBR is also used in everyday items, for example in footwear.
After synthetic rubbers, the other major use of butadiene is in the manufacture of 1,6-diaminohexane (hexamethylenediamine), which is then made into a polyamide.
Diaminohexane is made by passing hydrogen cyanide gas into butadiene in the liquid state, under pressure at ca 350 K.
A nickel compound is the catalyst for the reaction. The overall reaction is represented by the equation:

The resulting dinitrile is hydrogenated by passing its vapour and hydrogen over nickel at ca 500 K, under pressure (ca 35 atm):
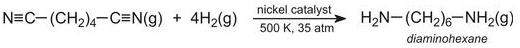
Annual production of butadiene
| World | 10.5 million tonnes1 |
| Asia Pacific | 6 million tonnes2 |
| North America | 3 million tonnes2 |
| Weatern Europe | 2 million tonnes2 |
Data from:
1. Merchant Research and Consulting, 2014. Expected to be 12.7 million tonnes in 2017.
2. Global Petrochemical Overview: Changing Olefins Markets, Nexant,2014
Manufacture of butadiene
It is mainly manufactured by the
- thermal cracking of naphtha (from oil)
- thermal cracking of butane (from natural gas and oil)
It is also made by the
- thermal cracking of gas oil (from oil)
Date last amended: 25th April 2017
