The best known manufactured polyamides are often called nylons (the trade name given by the manufacturer, DuPont) and these are aliphatic polyamides.
However, other manufactured polyamides are also important and these include an aromatic polyamide, Kevlar© and plastics produced from carbamide (urea). The nomenclature for describing the linear, aliphatic polyamides (the nylons) is based on the number of carbon atoms in the repeating unit.
| Polyamide (nylon) | Repeating unit |
|---|---|
| 6 | 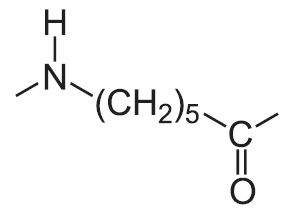 |
| 6,6 | 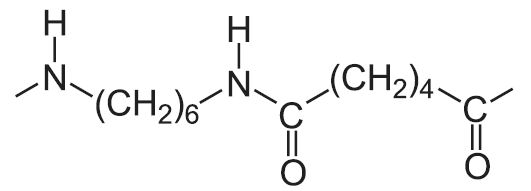 |
| 6,10 | 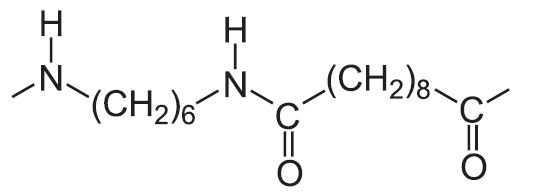 |
| 11 | 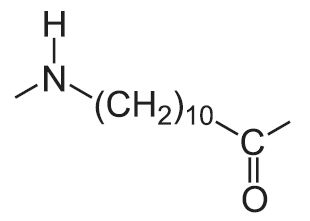 |
| 12 | 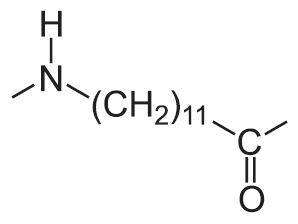 |
Uses of polyamides
Both polyamide 6.6 and polyamide 6 have a high tensile strength but polyamide 6.6 is able to absorb water and whereas polyamide 6 has enhanced elasticity. Both are tough and have resistance to abrasion.
They are produced both in solid form and as fibres.
The fibres, which account for more than half of the polyamides manufactured, are produced in a variety of forms, as textile filament (for clothing), as carpet filament and as industrial filament (for example, for ropes).
However, for either continuous filament or staple fibres, which are spun from the molten polymer at very high speeds (ca 6 km every minute), there is great emphasis on controlling the polymer chemistry and the way the yarn is produced in order to ensure the production of the high quality material needed for particular purposes. For example, the thread for use in stockings needs to be strong, as well as very fine, so the molecular mass and hence tensile properties of the polymer must be carefully controlled.
I want to find a photo of molten spinning
Although polyamides 6,6 and 6 account for 95% of the material used in women's hosiery, this still only accounts for about 5% of the total fibres used to make clothing. Nevertheless this is more than either the polypropenoates (acrylics) or wool but it is substantially less than either cotton or polyesters.
| Figure 1 The children's clothing is made of polyamide 6, impregnated with nanoparticles of titanium dioxide which gives protection against UV radiation, a very effective way of having a sunscreen. By kind permission of BASF. |
 |
The polyamides (nylons), in particular 6 and 6,6 are used in engineering plastics, for example, in cars. Polyamides 11 and 12 and also 6,10 are also used for this purpose.
The growth in the use of polyamides in recent years comes from their increasing use in the automotive industry (for example, battery casings, brake hoses, oil sumps and fenders). This reduces vehicle weight which in turn decreases fuel consumption and thus reduces pollution in the atmosphere.
When employed as an engineering plastic, polyamides are often used as co-polymers (for example co-polymers of 6 and 6,6) and compounded with other materials. These fillers include glass beads, glass fibres and carbon fibres. Pigments and fire retardants are also added.
In recent years a whole range of these polyamide mixes have been developed and marketed giving manufacturers using polyamides, a wider range of properties. Different co-polymers and different fillers with varying concentrations, are available depending on the property required, for example whether it is enhanced strength and rigidity, impact strength, elasticity or resistance to chemicals or heat, that is needed.
Polyamides are used as films for their good balance between mechanical strength and barrier properties against oxygen, smells and oils, for example for food packaging
| Figure 2 An important development is the use of polyamides to make safety airbags. By kind permission of the Delphi Automotive |
|||
 |
 |
Figure 3 Ropes made from polyamides are used by rock and ice climbers. They are not only very strong but they are also stretchy and thus reduce forces in the event of a fall, by spreading the duration of loading transmitted to anchors and to the body via the harness. Tony Moody is climbing ice on Heninger, near Cogne in northern Italy. By kind permission of Tony Moody |
Annual production of polyamides
Polyamides, Total
| World | 7.8 million tonnes1 |
| US | 0.6 million tonnes2 |
Polyamide 6,6
| World | 4.4 million tonnes1 |
Polyamide 6
| World | 3.4 million tonnes1 |
| China | 2.0 million tonnes3 |
Of the total of 7.8 million tonnes of polyamides produced in 2016, about 5.5 million tonnes were produced as fibres. This increased to 5.5 million tonnes in 20174. Almost all the US production of polyamides was converted into fibres.
1. In 2016 Plastics Insight, 2018
2. In 2014 2015 Guide to the Business of Chemistry American Chemistry Council 2016
3. In 2015 Kunststoffe International 10/2106
4. Statista 2018
Manufacture of polyamide 6 and 6,6
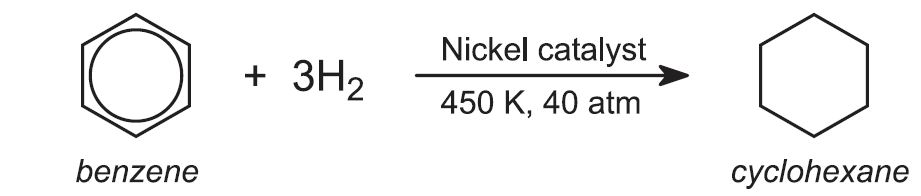
Both polyamides are manufactured from benzene via cyclohexane. Hydrogen is passed through liquid benzene in the presence of a nickel catalyst under pressure:
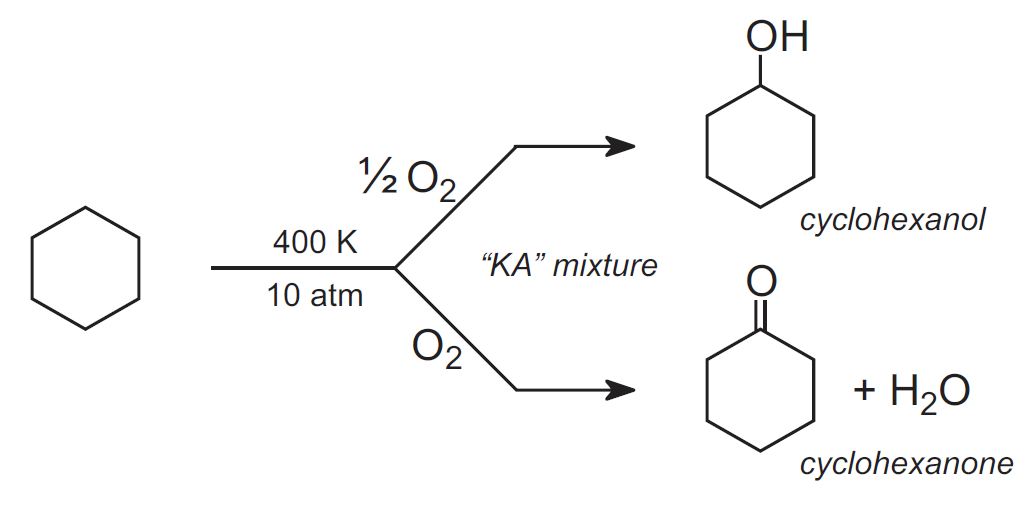
Cyclohexane is oxidized by passing air through the liquid under pressure in the presence of a catalyst (often a cobalt salt) to yield two products:
The mixture of cyclohexanol and cyclohexanone is known as "mixed oil" or KA (ketone/alcohol).
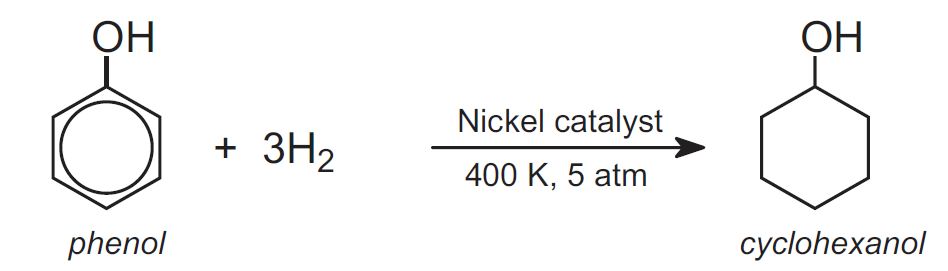
An alternative route to cyclohexanol is via the hydrogenation of phenol using a nickel catalyst at ca 400 K and 5 atm:
A more recent route to cyclohexanol is the Asahi process from benzene via its hydrogenation to cyclohexene and subsequent hydration to alcohol. This is more energy efficient than the other processes.
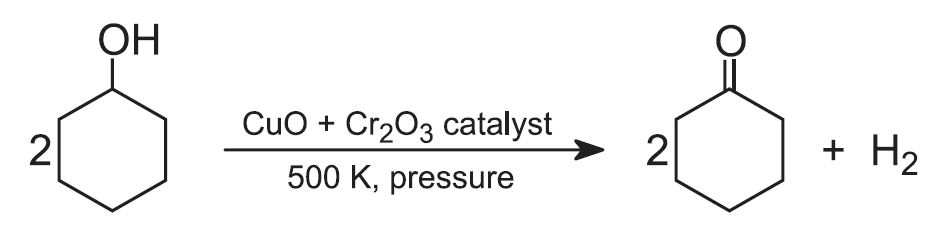
To make polyamide 6, pure cyclohexanone is required. When the mixed oil is heated under pressure with copper(ll) and chromium(lll) oxides, the cyclohexanol, which is a secondary alcohol, is dehydrogenated to the corresponding ketone, cyclohexanone:
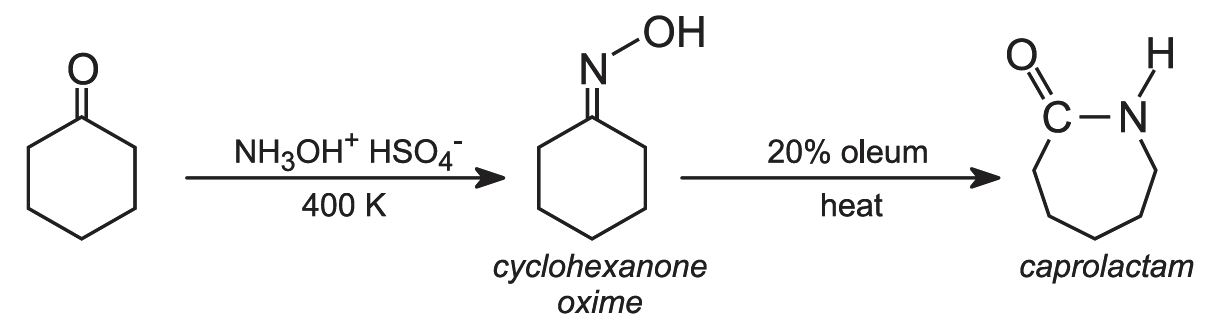
Cyclohexanone is then converted into caprolactam via the oxime (produced by the reaction of the ketone with hydroxylamine - in the form of the salt, hydroxylamine hydrogensulfate):
The isomerisation of the oxime to caprolactam by sulfuric acid is an example of the Beckmann rearrangement in which an oxime is transformed into an amide in the presence of acid.
A zeolite, with acidic sites, is also being used to effect the rearrangement. The zeolite is regenerated and saves the use of sulfuric acid.
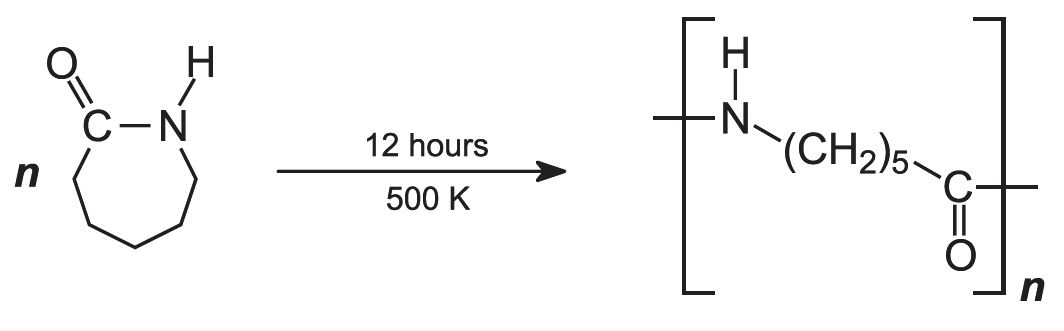
To produce the polymer, the caprolactam, water (acting as a catalyst) and a molecular mass regulator, e.g. ethanoic acid, are poured into a reaction vessel and heated under nitrogen at 500 K for about 12 hours:
This is an example of a batch process .
Polyamide 6,6 is produced by reacting 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) by condensation polymerization.
One of the monomers, hexanedioic acid is also produced from KA mixed oil (cyclohexanol and cyclohexanone). The mixed oil is oxidized in the liquid phase using moderately concentrated (60%) nitric acid and a copper(II) nitrate and ammonium vanadate(V) catalyst, at 330 K to form hexanedioic acid:
This process has a considerable disadvantage. A side-product is nitrogen(I) oxide (nitrous oxide), N2O, a powerful greenhouse gas but it is carefully removed by thermal or catalytic treatment units.
There are a variety of ways in which diaminohexane (hexamethylenediamine) is manufactured. Two important methods are described in other units. One uses buta-1,3-diene as the starting material, reacting it with hydrogen cyanide and hydroenaing the product. Another uses propenonitrile (acrylonitrile) as the starting material and diaminohexane is produced via a novel electrochemical process.
To form the polymer, the acid and the diamine are then heated together to form a salt.
The chemical reaction for aliphatic dicarboxylic acids and aliphatic diamines to yield an aliphatic polyamide via a condensation polymerization process can be represented, thus:
The chain length is regulated by controlling process conditions, such as reaction time, temperature and pressure. An aqueous solution of the salt is heated, in the absence of air, to ca 500 K. A pressure develops in the vessel. The temperature is then raised to 540 K, and the steam is bled off to keep the pressure constant. Eventually, the pressure is reduced and the polymer is extruded under nitrogen to yield a lace which is then granulated (Figure 4).
There have been attempts to make the process ‘greener’. One such development has been the production of polyamides which are made, at least in part, from renewable raw materials. An example is the production of polyamide 6,10. The 10 is the use of octanedioic acid (sebacic acid) instead of hexanedioic acid (adipic acid) in the reaction with the amine, 1,6-diaminohexane (hexamethylenediamine). Octanedioic acid is produced by the oxidation of castor oil, an oil made by extracting oil from the seeds of the Ricinus communis plant. These seeds are known as castor beans.
Other polyamides
Other important polyamides include the aramid Kevlar© and the carbamide-methanal and melamine-methanal plastics.
| Figure 4 The granules are a polyamide from which the frames of the glasses have been moulded. By kind permission of Arkema. |
 |
Date last amended: 8th November 2018