.jpg) Methanol is produced from synthesis gas (carbon monoxide and hydrogen), itself derived from oil,coal or, increasingly, biomass. It may become central to the development of biorefineries as an intermediate in the conversion of biomass to useful products.
Methanol is produced from synthesis gas (carbon monoxide and hydrogen), itself derived from oil,coal or, increasingly, biomass. It may become central to the development of biorefineries as an intermediate in the conversion of biomass to useful products.
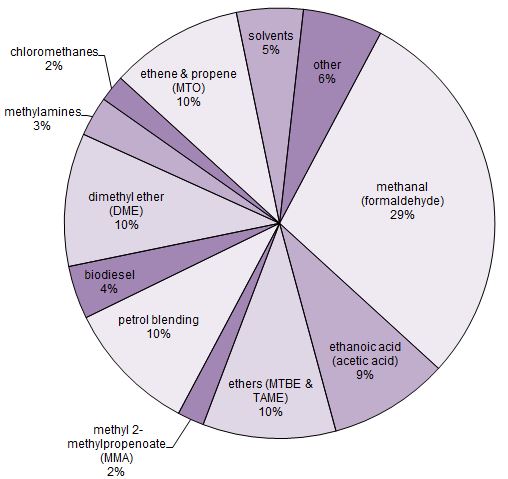
Uses of methanol
Figure 1 Uses of methanol.
Data for 2015, from IHS Markit, 2015
(a) Polymers
The largest use for methanol is as a feedstock for the plastics industry.
(i) Plastics derived from methanal
Methanol is used to make methanal and hence a variety of plastics, based on reactions with phenol, carbamide (urea) and melamine.
(ii) Polyesters
The production of polymers such as the polyester Terylene use methanol as the original feedstock.
Methanol is used to make Terylene in two ways. One is as the alcohol to make the dimethyl ester of benzene-1,4-dicarboxylic acid (terephthalic acid). The other is to make ethanoic acid (acetic acid), a large amount of which is used in the manufacture of benzene-1,4-dicarboxylic acid. It is used as the solvent in the liquid phase oxidation of 1,4-dimethylbenzene (p-xylene), leading to the production of the acid.
(iii) Poly(methyl 2-methylpropenoate)
Poly(methyl 2-methylpropenoate) is used under trade names such as Lucite, Perspex and Altoglass. Methanol is used to make the monomer, the methyl ester of 2-methylpropenoic acid.
(iv) Poly(ethene) and poly(propene)
A most remarkable increase is in the use of methanol to produce alkenes by the MTO and MTP processes, from 6 million tonnes in 2015 to an expected 20 million tonnes in 2020, which will mean that a large proportion of plastics such as poly(ethene) and poly(propene) will, in the near future, be derived from synthesis gas.
(b) As a fuel
Methanol is destined to be a major fuel for cars, either as a liquid fuel, mixed with petrol, or in fuel cells where it has been used to prepare, in situ in the car, hydrogen for the fuel cell.
Whereas a few years ago, only a small amount of methanol was used directly as a fuel in cars, this use is now increasing rapidly. In China petrol is mixed with methanol (15%) without the need for engines to be redesigned. With some redesigning, more methanol (up to 85%) can be used. The advantage for China is that the methanol can be produced from both coal and biomass via synthesis gas. This emphasis of using methanol as a fuel is reflected in the global production figures for methanol. Thus worldwide, use of methanol as a fuel now accounts for 10% (about 7 million tonnes a year) but is expected to increase in the coming years.
China's use of methanol as a fuel increased 25% annually from 2000 to 2015.
|
||||||
 |
| Figure 4 Stena Line is converting its fleet of ships to run on methanol in order to reduce the pollution that occurs when burning fuel oil which is of particular concern in the Baltic. The Stella Germanica, here in Gothenburg, Sweden, getting ready to sail to Kiel in Germany, underwent extensive sea trials using methanol before the decision was made to convert over 20 other ships. By kind permission of Marcusroos (Wikimedia Commons). |
(c) To make fuels
(i) The MTG process
Synthesis gas can be converted into liquid fuels. One way is by using the Mobil MTG (methanol to gasoline) process.
Methanol is converted into alkanes and aromatic hydrocarbons suitable for petrol (hydrocarbons with 5 to 8 carbon atoms), by passing the vapour over alumina at ca 600 K. An equilibrium mixture of methanol, dimethyl ether (DME) and steam is produced, containing about 25% methanol:
.jpg)
This mixture of gases is then passed over a bed of a zeolite in its acid form, HZSM-5, heated to ca 650 K, to produce the mixture of hydrocarbons (with 5-10 carbon atoms) for use as petrol.
DME can be used in a different way. Although it is a gas at ambient temperatures, it can be readily liquefied under pressure and is considered to be an attractive alternative fuel to diesel oil. The vehicles need a compression ignition engine with a fuel system specifically developed to operate on DME. There have been a number of DME vehicle demonstrations in Europe and in the US, including one in which a customer operated 10 vehicles for 750,000 miles. Emission standards for particulates can be met without the use of filters. As with conventional diesel vehicles, oxides of nitrogen (NOx) emissions can be reduced in the usual way with a solution of urea.
DME is also mixed with liquid petroleum gas (LPG) as a fuel for use in homes. Its main use at present, though, is as an aerosol propellant.
The first MTG plant was built in New Zealand and new plants are now being built that will also respond to demand for methanol and ammonia, both of which need synthesis gas.
This processes can provide a route to produce chemicals from biomass. The biomass is converted to synthesis gas, then to methanol and thence to liquid fuels.
(ii) To make oxygenates
Another important use of methanol is in the manufacture of methyl t-butyl ether (MTBE) and t-amyl methyl ether (TAME), additives to petrol to increase its octane rating.
However, MTBE has been shown to be a serious pollutant and when spilled gets into water courses. Its use is being phased out in the US and in other countries.
Annual production of methanol
| World | 70 million tonnes1,2 |
| Asia | 44 million tonnes3 |
| Middle East | 9 million tonnes3 |
| US | 2 million tonnes4 |
1. M Alvarado, Methanol, 2016, IHS
2. Expected to be approaching 80 million tonnes in 2016 and 100 million tonnes in 2020
3. Methanol Market Services Asia, 2016. Data estimated for 2015
4. 2015 Guide to the Business of Chemistry, American Chemistry Council, 2016
In 2000, China accounted for about 12% of the world's consumption of methanol while North America and Europe consumed 33% and 22% respectively. By 2015, China consumed 54% while North America and Europe consumed 11% and 10%.
Manufacture of methanol
(a) Production of synthesis gas
(i) Traditional methods
Methanol is manufactured from synthesis gas which is a mixture of carbon monoxide and hydrogen.
The feedstock, over the last 40 or more years, has been oil or natural gas. Coal, particularly in China, coal, rather than natural gas or oil, is being used as the feedstock.
(ii) ‘Green’ methanol
There have been major developments to produce methanol which is largely ‘green’.
Any solid biomass including for example agricultural, city and industrial waste can be used to make synthesis gas using techniques similar to its production from coal.
More recent developments include a plant in the Netherlands, which is using liquid propane-1,2,3-triol (glycerol), a by-product from the production of biodiesel, from animal fats and vegetable oils, to produce the gas.
Another ‘green’ route is to use waste carbon dioxide. Although the first such plant is linked to geothermal energy, it could be used to convert carbon dioxide waste from, for example, lime kilns and steel manufacture, to methanol.
(b) Synthesis of methanol
Synthesis gas is catalytically converted to methanol at elevated temperatures and pressures in a fixed bed reactor. The catalyst is an alumina pellet coated with copper and zinc oxides.
The main methanol synthesis reaction may be written:
![]()
From considering the energetics of the reactions, it can be seen that the yield of methanol is favoured by high pressures and low temperatures. A low pressure process came about by the discovery of a copper-based catalyst which was active at 475-575 K, thus allowing economical conversions to occur at 40-100 atm. One plant, for example, operates at 525- 575 K and 100 atmospheres. It eventually achieves a 97% conversion of the reactants.
The actual mechanism for the formation of methanol has been an active area of research. By using radioactive 14CO2 it is believed that the majority, if not all, of the methanol is derived via CO2.
.jpg)
Figure 4 The converter in which methanol is being produced from synthesis gas.
By kind permission of Johnson Matthey.
Date last amended: 17th February 2017

.jpg)
.jpg)