.jpg) Except for helium, which is mostly extracted from natural gas, oxygen, nitrogen and the other rare gases are extracted from the air that makes up Earth's atmosphere. Unlike the sources of some chemicals there are therefore no concerns about the depletion of this resource and if a sample of air is used to produce one of the gases, there are no problems about venting the 'waste gases' back into the atmosphere.
Except for helium, which is mostly extracted from natural gas, oxygen, nitrogen and the other rare gases are extracted from the air that makes up Earth's atmosphere. Unlike the sources of some chemicals there are therefore no concerns about the depletion of this resource and if a sample of air is used to produce one of the gases, there are no problems about venting the 'waste gases' back into the atmosphere.
The ready availability of oxygen and its reactivity with many other elements means that it is used during the production of many other chemicals, whereas some of the uses of nitrogen and the rare gases depend on their inertness.
Uses
Oxygen
The largest user of oxygen is the steel industry. It is also used in the manufacture of other metals, notably copper and lead. It is more economic to use pure oxygen, or oxygen-enriched air, rather than air as this increases the reaction rates and means that smaller chemical plants can be used. Further it makes it easier to ensure that no gases such as sulfur dioxide are lost and pollute the atmosphere.
The gas is also used in the manufacture of many chemicals including nitric acid, hydrogen peroxide, epoxyethane and chloroethene (vinyl chloride), the precursor to PVC.
Among its other uses is the burning off of carbon deposited on the fluid catalyst used in the catalytic cracking of gas oil
A growing use for oxygen is in treating sewage and effluent from industry. Polluted rivers and lakes can be cleaned by dissolving oxygen gas directly into the water to encourage a better ecological balance. It is used, for example, in fish farming to provide this balance.
It is also used, with sodium hydroxide, to bleach paper pulp as an alternative to chlorine dioxide or sodium chlorate(I) (sodium hypochlorite).
Nitrogen
Nitrogen is used to make ammonia. It is also widely used to provide an inert atmosphere, a process known as 'blanketing', principally to exclude oxygen. For example, nitrogen is used in this way in food packaging, glass making, and semiconductor manufacture. It is also used to purge out pipes prior to welding (for example, oil pipes) to ensure that no flammable vapours are left behind.
Liquid nitrogen is being increasingly used to refrigerate food during transportation. Medical samples containing, for example blood, viruses for vaccinations and semen, can be stored for long periods if kept cool in liquid nitrogen.

Figure 1 Oil tankers are purged with nitrogen to prevent explosions during welding and other
maintenance. The photo is of the Omala moored in Rotterdam, the Netherlands.
By kind permission of Danny Cornelissen (Wikimedia Commons).
Rare gases
Liquid helium, with its very low boiling point of 4 K, has become very important in cooling superconductor magnets, used, for example, in MRI scanners for medical diagnosis. The magnets generate much heat and helium is the first choice as it has the lowest boiling point of any liquid. Its inertness is fundamental to its other major uses, for example, to provide an inert atmosphere when welding metals (to prevent metals reacting with air to form oxides and nitrides). It is also used to purge gas from tanks which have contained fuels such as liquid hydrogen.
It also provides an inert atmosphere during the manufacture of high value materials such as optical fibres.
Due to its low density, helium is used for weather balloons and airships. It is also used in combination with oxygen to produce specialised breathing mixtures for divers. These mixtures are better than nitrogen-oxygen mixtures for prolonged diving as the blood will absorb only a limited amount of helium whereas with nitrogen, its concentration can build up to dangerous levels over a prolonged period.
Helium is also used in eximer lasers and helium-neon lasers that are used for bar code scanners.
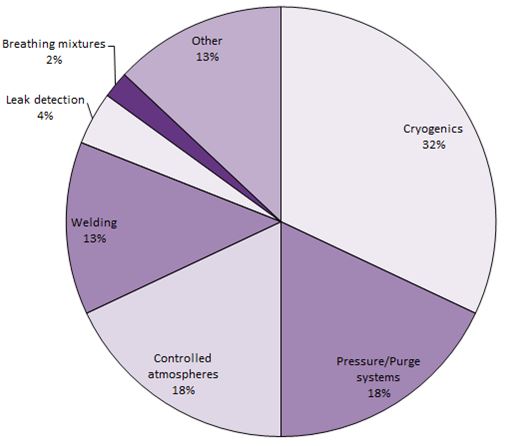
Figure 2 Uses of helium in the United States
Data for 2011 from USGS
The most common use for neon is in electric discharge lamps, i.e. fluorescent tubes. Like krypton and xenon, neon emits light when an electric current is passed through the gas at low pressures. Neon provides a red or orange colour, while different mixtures of neon, argon and helium can be used to achieve white, mauve, yellow, blue and green. It is also used in lasers (helium-neon lasers for laser pointers) and in plasma display panels.
All of the major uses for argon are related to the production, processing and fabrication of metals. Argon is a significant shielding gas used during arc welding processes, either on its own or combined with other gases. It is also used as the inert gas in electric light bulbs (when mixed with nitrogen), as a low thermal conductivity medium within double-glazing window units and for semiconductor manufacture.
|
Figure 3 The closing ceremony of the London Olympics, August 12th 2012. The stadium is illuminated by metal halide floodlights. The lamps contain argon which helps the start up. The heat from this start up evaporates the metal halide which then provides the visible light. |
 |
Krypton is not only used in fluorescent lamps but also in incandescent lamps including flash bulbs and halogen lamps which are used, for example, in some cars. It is also found in some eximer lasers and in plasma display panels.
Xenon is, like other rare gases, used in bulbs, including halogen lamps. It has a light spectrum that is much wider than the other rare gases and appears to the human eye as daylight and so is used in lighting large areas such as sports stadiums, stage lighting and airport runways.
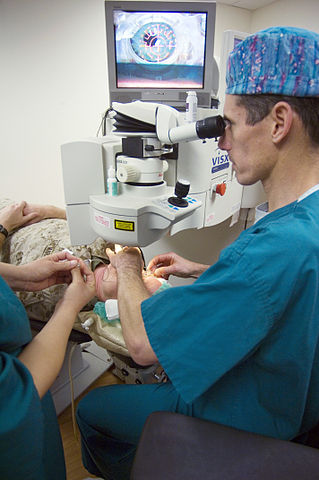
Xenon, mixed with other rare gases is used in eximer lasers for surgery (for example in eye surgery).
Mixed with oxygen, it is used for CAT (Computer Aided Topography) scanning for mapping blood flow.
Xenon is also being used as an anaesthetic.
| Figure 4 An opthalmology surgeon at the US National Medical Center, Bethesda lines up an excimer laser on a patient's eye before beginning laser surgery. By kind permission of the US Navy (Wikimedia Commons) |
 |
Annual production
About 5 million tonnes of oxygen and nitrogen (excluding that produced for the manufacture of ammonia) are produced annually in the UK.
In comparison, much smaller amounts of the rare gases are produced worldwide (Table 1).
| Rare gas | Abundance in the atmosphere/ppm | World production/tonnes per year |
|---|---|---|
| helium | 5,2 | 28 0001 |
| neon | 18 | 12 |
| argon | 9300 | 700 0002 |
| krypton | 1.1 | 82 |
| xenon | 0.08 | 0.62 |
Table 1 Rare gases: their abundance in the atmosphere and world production.
| Data from: 1 U.S. Geological Survey, Mineral Commodity Summaries, 2016 2 enviromentalchemistry.com |
Manufacture
Air contains nitrogen, oxygen and argon with traces of other gases, including water vapour. Although the presence of carbon dioxide in air is very important environmentally, it accounts for less than 0.04%. On average, the composition of dry air is, by volume:
| Gas | % |
|---|---|
| nitrogen | 78 |
| oxygen | 21 |
| argon | 0.95 |
Table 2 Composition of dry air.
Carbon dioxide, helium, neon, krypton and xenon make up the balance.
There are several different methods for manufacturing nitrogen and oxygen:
a) Distillation of liquid air at cryogenic (very low) temperatures. This produces large volumes of very pure (up to 99.95%) oxygen, nitrogen and the rare gases. Oxygen and nitrogen are often transported as liquids (by road tanker) and gases (by pipeline) to where they are to be used on a large scale. Sometimes, however, the plant is built where the gases are being used (for example in the manufacture of steel).
b) Pressure swing adsorption. This produces either nitrogen and oxygen, where it is to be used (on site), in smaller quantities and up to 95% purity. The main impurity is argon.
c) Vacuum swing adsorption. This is used to produce oxygen on site, up to 95% purity. The main impurity is argon.
d) Membranes. These can also produce nitrogen on site in small amounts with purity of 95 to over 99%.
The choice of method depends on the use of the gas and the amount needed.
(a) Manufacture of nitrogen and oxygen by cryogenic separation of air
There are several different stages in the process (Figure 4), the three key ones being:
i) cleaning the air
ii) liquefying the air
iii) distillation of the liquid air
(i) Removing impurities from air
In a typical plant, air is drawn in through a filter to remove the dust, compressed to about 6 atm and cooled to below ambient temperature where much of the water vapour condenses. The air is then passed through a zeolite molecular sieve to remove the rest of the water vapour and carbon dioxide.
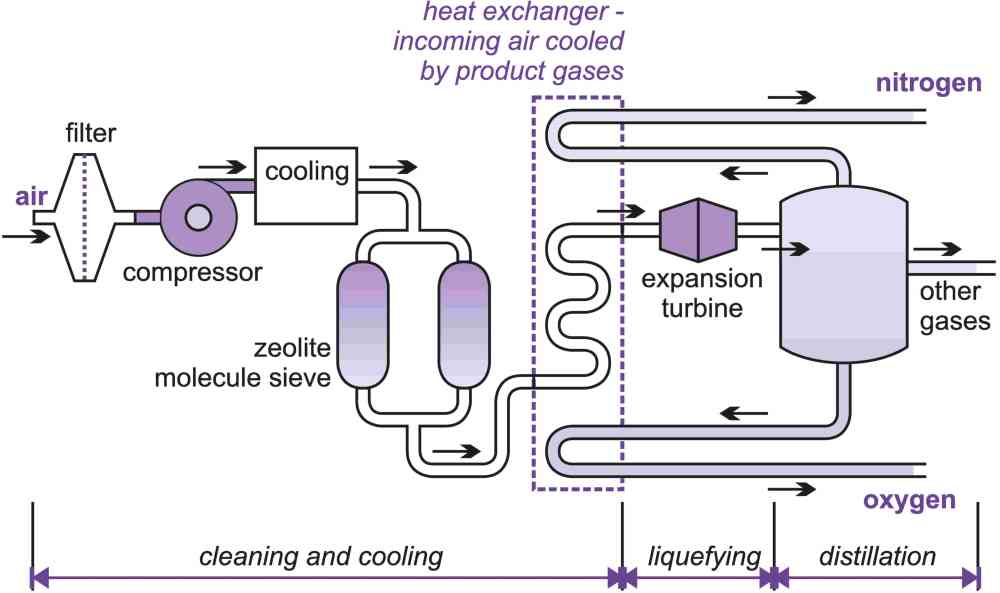
Figure 5 Key stages in the manufacture of nitrogen and oxygen.
(ii) Liquefying the pure air
The manufacture of nitrogen, oxygen and argon from atmospheric air involves liquefying the air and then separating it into its component parts by fractional distillation. Since nitrogen and oxygen have very low boiling points (Table 3), they liquefy at cryogenic temperatures.
| Constituent | Boiling Point/K, at atmospheric pressure |
|---|---|
| helium | 4 |
| neon | 27 |
| nitrogen | 77 |
| argon | 87 |
| oxygen | 90 |
| krypton | 120 |
| xenon | 165 |
| carbon dioxide | 195 (sublimes) |
Table 3 The boiling points of the gases that make up dry air.
When a bicycle tyre is pumped up, from atmospheric to a higher pressure, heat is produced. Conversely, if gas at high pressure is expanded to a lower pressure, there is a drop in temperature. Almost all methods of producing low temperatures depend on this property but it is not a very efficient technique. A more efficient way is expansion in a turbine, where mechanical work is extracted. Air separation plants use one or both of these methods, in conjunction with heat exchangers to achieve cooling.
The pure air, already compressed to 6 atm, is cooled with the very cold product and the waste (nitrogen if oxygen is being made and oxygen, if nitrogen is being made) in a series of heat exchangers.
Eventually, this compressed air is cooled to about 100 K and then allowed to expand rapidly (the expansion turbine) which cools it further until it is liquefied.
The key to the whole process is this use of the very cold nitrogen, oxygen and argon to cool the incoming air prior to the expansion.
(iii) Distillation of liquid air
The liquid air is separated into its constituent components by fractional distillation. At each distillation the vapour is richer in nitrogen (the component with the lower boiling point), while the liquid remaining contains more oxygen (the component with the higher boiling point).
To produce pure oxygen the distillation system has two distillation columns, a 'high' and a 'low' column. Nitrogen plants often use a single column but some use two.
Nitrogen leaves the top of the column as a gas. Purity levels required by nitrogen users are becoming increasingly stringent and it may be liquefied again and redistilled. It is unusual for the nitrogen to have an oxygen content of more than 10 ppm and impurities contained in nitrogen for the electronics industry are measured in parts per billion.
|
The liquid containing oxygen and argon is further purified by distillation in a second column. To obtain high purity oxygen, further distillation is needed and argon is removed.
Since air separation plants operate at low temperatures, construction materials have to be chosen carefully. Aluminium alloys and stainless steel are frequently used. They do not become brittle at low temperatures.
Efficient plant insulation is necessary to make the process economic and safe. Perlite (an expanded rock), glass wool and vacuum jacket techniques are commonly used (Figure 5).
(b) Manufacture of oxygen and nitrogen using pressure swing adsorption (PSA)
It is sometimes more convenient for some industrial plants to produce their own oxygen and nitrogen on site, provided a purity of 90% or so is acceptable. Pressure swing adsorption can provide this and produce up to about 100 tonnes a day.
To produce oxygen, a stream of clean air is passed through a bed of alumina to dry the gas and then through a bed of zeolite molecular sieve. Nitrogen is preferentially retained (adsorbed) leaving an oxygen-enriched gaseous stream to pass through (Figure 7). When the zeolite becomes saturated with nitrogen it is necessary to regenerate it. This can be achieved simply by reducing the pressure, whereupon the nitrogen is released (desorbed) back into the gaseous phase and rejected as waste. The sieve is totally regenerated in this way and is ready to repeat the cycle.
Two beds are usually used in rotation. One is used to produce the oxygen while the other is being regenerated. The full cycle time can vary between 2 and 8 minutes depending upon actual performance requirements.
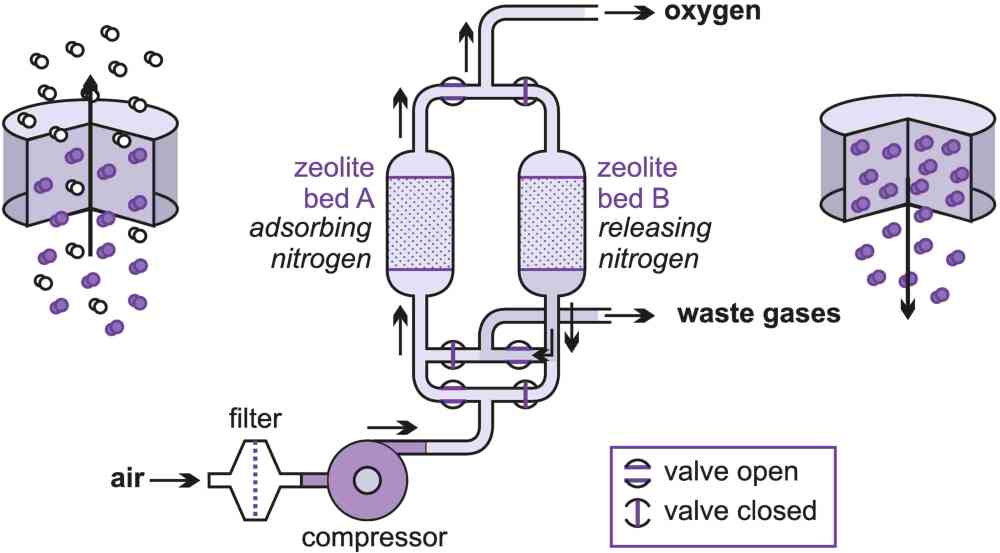
Figure 7 Purification of oxygen using pressure swing adsorption.
Instead of using a zeolite as in the manufacture of oxygen, nitrogen is produced from air using beds of carbon molecular sieve (CMS). Clean, dry, compressed air is passed through a bed of CMS (typically at 7-I2 atm). Oxygen is adsorbed on the surface of the CMS and nitrogen passes through to storage. When oxygen saturation is reached a second bed is brought on-stream, retaining continuity of supply and the first vented to atmosphere to desorb the oxygen and hence regenerating the CMS prior to the next cycle. Similar to the production of oxygen by PSA, the full cycle time for nitrogen varies between 2 and 8 minutes.
(c) Manufacture of oxygen by vacuum swing adsorption
Oxygen can be produced on site by a process very similar to pressure swing adsorption except that the sieve material is regenerated by placing it under vacuum. This process is more expensive to build but it is more efficient to run. The sieve is more effectively regenerated and as a result, more oxygen is obtained from a given amount of air.
(d) Manufacture of nitrogen using membranes
The principle underlying this method is that gases diffuse at different rates through a polymer film membrane. Oxygen and carbon dioxide diffuse more rapidly than nitrogen and argon and this allows the remaining gas to become richer in nitrogen and argon. Eventually, the nitrogen concentration becomes over 95%.
The polymer used for the membrane is often made of poly(methylpentene). Air, at pressures of between 7 and 12 atm and heated to 290 to 310 K, is passed through the membranes. The waste gases permeate through the membrane and are vented to the atmosphere.
(e) Manufacture of rare gases
Argon is obtained during the cryogenic manufacture of nitrogen and oxygen, using a separate distillation column mounted alongside the second (low pressure) column used to purify oxygen.
At this point in the distillation process, the feed is typically 89% oxygen and 11% argon with only traces of nitrogen and is re-distilled to obtain argon of approximately 98% purity, known as Industrial Argon. When very high grade (99.999%) argon, Pure Liquid Argon (PLAR), is needed, industrial grade argon is processed in a separate plant, the Argon Purification Unit. This plant removes residual oxygen by mixing the gas stream with hydrogen and passing the mixture over a catalyst. Oxygen combines with hydrogen and the water formed is removed by passage through a molecular sieve. Residual nitrogen is then removed by further distillation at cryogenic temperatures.
Neon (boiling point 27 K) does not condense out at the temperatures used in air separation plants and is withdrawn, with helium, and cooled to liquid nitrogen temperature. The helium is removed by adsorption on activated charcoal.
Krypton and xenon (boiling points 120 and 165 K respectively) accumulate in the liquid oxygen and are obtained by further distillation.
Helium is mostly obtained from natural gas. Particularly large amounts are found in the US, Qatar, Algeria, Russia (Siberia and Eastern Russia), Australia and Canada. Some of the natural gas deposits in the US contain 16% helium. Natural gas is compressed (40 atm) and the condensed materials are removed. Hydrogen sulfide is removed by washing with 2-aminoethanol, as in the sulfur extraction process and water vapour is removed by passing gas through specially treated alumina. The gas is further purified by a three-stage cooling process, purer helium being removed after each stage and passed on to the next cooling step. Helium is available in 99.95% and 99.995% purity grades.
Date last amended: 12th October 2016