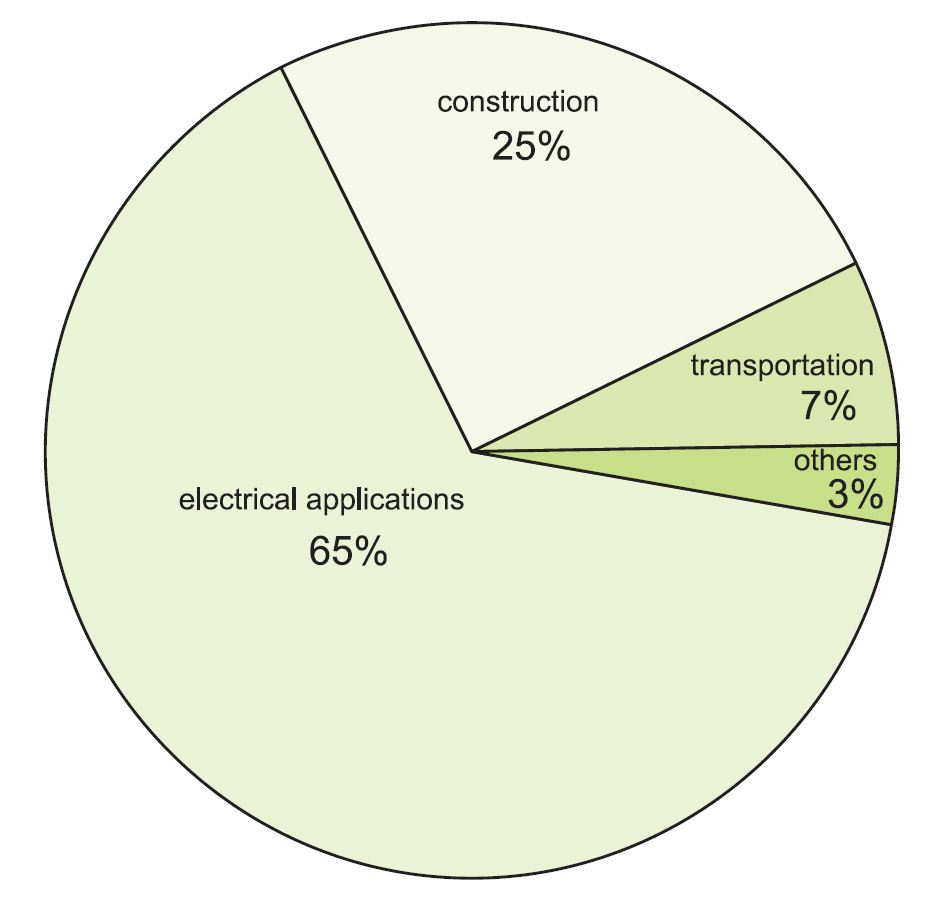
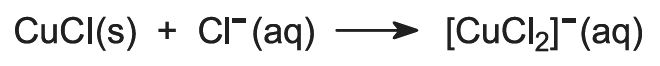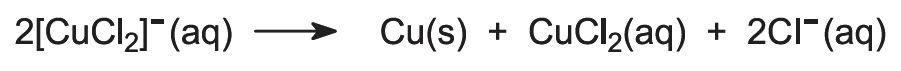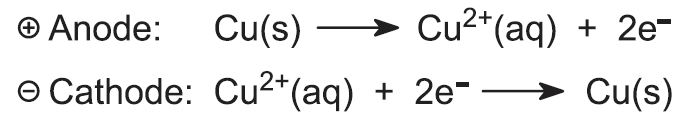Uses of copper
Figure 1 Uses of copper.
 |
| Figure 2 The Statue of Liberty was recently renovated after suffering over a century of weathering. The copper skin was intact, only the torch needed attention. By kind permission of The Copper Development Association. |
In the UK, copper is mainly used to make semi-finished products (called semis) which are made from the refined metal, either as pure copper or as copper alloys. They can be in the form of wire, rod, bar, plate, sheet, strip, foil or tube. Over half the copper is sold in the form of cables, wire and tubes. Much of the rest is made into alloys.
By far the largest proportion of copper is used globally in electrical wiring, printed circuit boards, in generators, electric motors and transformers. An average car, for example, has about a mile of copper wiring, with a mass of 1 kilogramme. The new Boeing 787 (the Dreamliner) has some 120 miles of wiring with a mass of 4 tonnes.
Copper is also used in cars in various electronic devices, such as sensors to monitor and control temperature and speed.
Much copper is used in buildings, not just in copper piping and wiring, but also in cladding, which results in a very attractive colour. It is also used in refrigerators and air conditioning units for its ease of fabrication and its thermal properties.
Annual production (Primary copper)
| World | 18.9 million tonnes1 |
| Chile | 5.7 million tonnes2 |
| China | 1.8 million tonnes2 |
| Peru | 1.6 million tonnes2 |
| US | 1.8 million tonnes2 |
| Congo (Kinshasa) | 1.0 million tonnes2 |
Data from:
1 International Copper Study Group, 2015.
2 U.S. Geological Survey, Mineral Commodity Summaries, 2016.
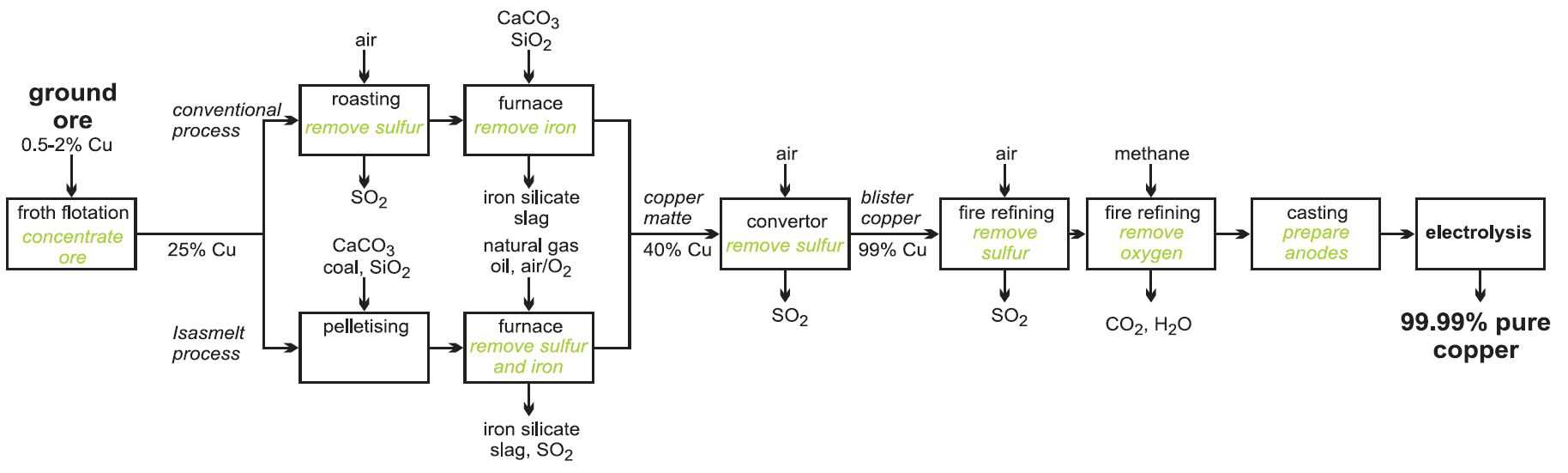
Manufacture of copper
About 80% of the world's primary copper comes from ores in which copper is present as a sulfide mineral, for example, chalcopyrite (CuFeS2) (the most abundant copper ore), bornite (Cu5FeS4) and chalcocite (Cu2S). These ores contain typically only about 0.5-2% copper. The remainder of primary production comes from ores in which copper is present as silicates, sulfates, carbonates and oxides, which have been formed by the weathering and oxidation of sulfide minerals. About 30% of all copper production is recovered from secondary and scrap materials which are recycled.
Major deposits of the ores are in Chile, the western part of the US, Canada, Zambia, the Democratic Republic of the Congo and Russia.
Figure 3 The Bingham Canyon mine in Utah in the United States. It is the largest open cast copper mine in the world.
By kind permission of Tiffany Beveridge.
The manufacture of the copper takes place in three stages:
a) concentration of the ore
b) conversion of the sulfides and other copper compounds to copper
c) purification of copper
(a) Concentration of the ore
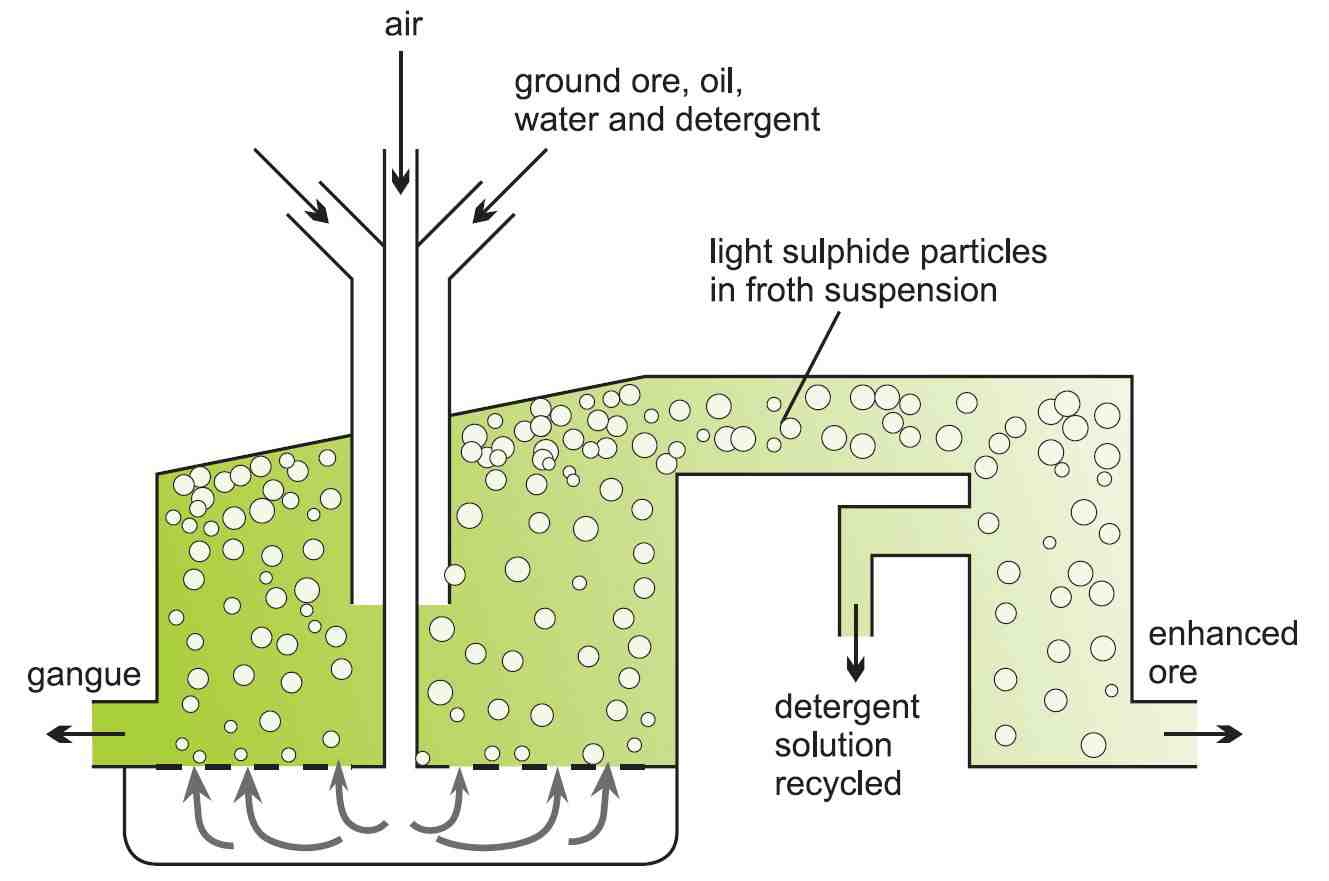
The ore is enriched by froth flotation (Figure 4). The powdered ore is mixed with oil and agitated with water in a large tank to which detergent has been added.
Compressed air is forced through the mixture, and the lightweight particles of copper sulfide are carried to the top and float on the froth. Heavier clays and other silicates settle to the bottom of the tank. This residue is known as 'gangue'. The copper-laden froth is skimmed off.
Figure 4 Concentration of copper ore by froth flotation.
(b) Conversion of the sulfides and other copper compounds to copper
The conversion is done by several methods:
i) by roasting copper sulfide ores
ii) the leaching process
iii) the bacterial method
(i) By roasting copper sulfide ores
The enriched ore is roasted with just enough air to convert the iron sulfide to iron(ll) oxide:
The solid mixture is then mixed with calcium carbonate (limestone), silica (sand) and heated to 1300 K. The iron forms a silicate slag and the copper(I) sulfide melts and sinks to the bottom of the furnace. It is known as copper matte.
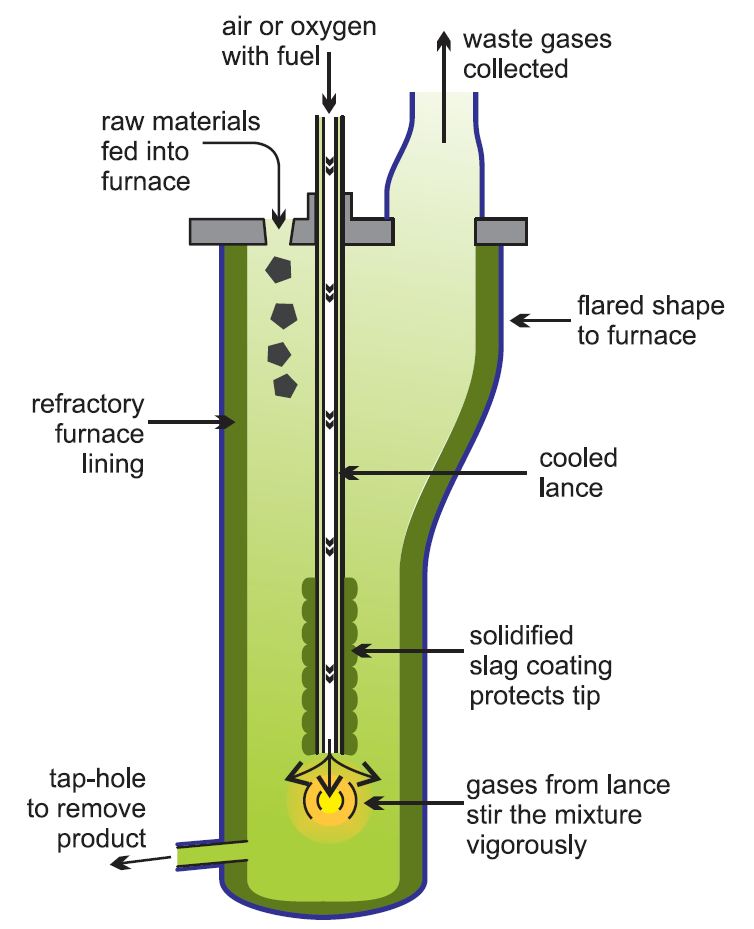
In a recent development, the Isasmelt process, the enriched ore (the concentrates), limestone and silica together with a solid fuel (coal) are mixed and pressed into pellets. These are fed into a furnace in which there is a lance down which natural gas (methane) and oil with oxygen-enriched air are pumped. It is more economic to use pure oxygen, or oxygen-enriched air, rather than air as this increases the reaction rates and means that smaller chemical plants can be used and fuel cost are reduced. Further it makes it easier to ensure that no gases such as sulfur dioxide are lost and pollute the atmosphere. Oxygen plants are constructed on the site.
Figure 5 The manufacture of copper using the Isasmelt process.
This mixture is pumped down at speed which causes turbulence and promotes a very rapid reaction. The process is highly efficient and a large amount of raw material can be processed in relatively small furnaces.
The copper matte and slag are tapped into another furnace for settling and separation.
The copper matte is then run into another furnace and air or oxygen-enriched air is blown in, to produce copper metal:
The sulfur dioxide is often converted, on site, to sulfuric acid.
This impure copper (ca 99%) is known as blister copper. It is heated until it is molten and more air is injected in to remove unwanted sulfur. This is followed by the injection of methane to remove oxygen. The process is known as fire-refining. The still impure copper is then cast into anodes for electro-refining.
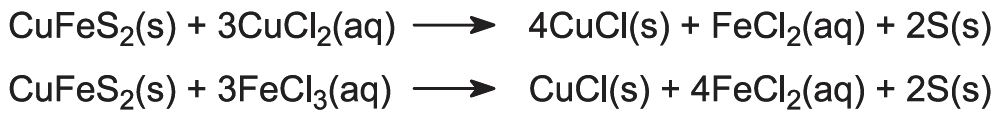
(ii) The leaching process
Copper is obtained from its ore by treating the ore with a solution of copper(ll) chloride and iron(lll) chloride:
Copper is recovered in the form of copper(l) chloride. To keep the compound in solution, sodium chloride is added. In the presence of excess chloride ion, the complex ion [CuCl2]- is formed, which is soluble in water:
Finally, impure copper is obtained by electrolyzing the solution of the [CuCl2]- ions to the metal:
Copper(ll) chloride is then recycled.
(iii) The bacterial method
A significant amount of the copper produced in the US is obtained by using bacteria. Acidified water is sprayed onto copper-mining wastes, which contain low levels of copper. As the water trickles down through the crushed rock, the bacterium Thiobadllus ferrooxidans, which thrives in the presence of acid and sulfur, breaks down the iron sulfides in the rock and converts iron(ll) to iron(lll) ions. The iron(lll) ion in turn oxidizes the sulfide ion of copper sulfides to the sulfate, leaving copper(ll) ion in solution. This copper-laden water is recovered at the bottom of the pile, and metallic copper is obtained by reduction with scrap iron:
(c) Purification of copper
Whatever method is used to manufacture copper from its ore, its final purification is by electrolysis (Figure 6).
Slabs of impure copper (blister copper), together with thin sheets of pure copper metal or stainless steel or titanium are immersed in a solution of copper(ll) sulfate (0.3 mol dm-3) and sulfuric acid (2 mol dm-3). The pure copper or steel sheets make the cathode (Figure 7) of an electrolysis cell, and the impure slabs are the anode. This means that copper ions are formed at the anode (oxidation occurs) and move into solution:
Figure 6 Purification of copper by electrolysis.
The ions migrate to the cathode, are reduced to pure copper and deposited on the cathode. From time to time, the pure copper is scraped off the cathode.
Many impurities from the copper anode, such as gold, silver, platinum and tin, are insoluble in the electrolyte solution and so do not deposit on the cathodes. Instead, they are deposited as 'anode slime' on the bottom of the tank, which is periodically removed and sent to specialist refiners. Other metals, e.g. iron and nickel, are soluble, so the electrolyte has to be continuously purified to prevent excessive deposition of these elements onto the cathode. Copper of purity of at least 99.99% is obtained in this way.
The copper obtained will then be made into convenient shapes (such as sheet, wire, rod, tubes etc) for use in manufacturing.
Figure 7 Pure copper cathodes at a refinery in Canada.
By kind permission of Anglo American.
Secondary production
Copper and alloys with a high copper content are recycled to obtain pure copper. The metals are heated with oxygen-enriched air, which oxidizes most of the metals, but not the copper or any precious metals, forming a slag that can be removed. The Isasmelt process described above is often used for secondary copper production.
The remaining copper, now about 99% pure, is cast into anodes and purified further using the electrolytic method described above.
Globally, it is estimated that about 33% of new copper products are made from recycled copper with some countries having significantly higher rates of recycling, for example in the North America (31%) and countries in Western Europe (47%). About half of this comes from scrap from mills and foundries making copper articles which is then simply remelted and cast. The other half comes from 'old scrap', for example from electric cables and plumbing.
Data from:
The World Copper Factbook, 2015; International Copper Study Group
Figure 8 A flow diagram summarising the processes used to manufacture copper from its ore.
Copper alloys
Many commonly used alloys contain copper as the predominant metal, with varying quantities of other elements (Table 1).
| Alloy | Copper is alloyed with | Uses |
|---|---|---|
| Brass | zinc | screws, wires, plumbing components, electrical connectors, musical instruments, door furniture and ornaments |
| Bronze | tin | statues, bearings, electrical connectors, springs, clips |
| Phosphor bronze | tin, phosphorus | precision bearings, springs, cymbals, instrument strings |
| Aluminium bronze | tin, aluminium (iron, nickel, silicon) | tools, high-temperature aircraft and automobile engine components |
| Cupronickel | nickel (iron, manganese) | coins, external components in marine environments |
| Nickel Silve | nickel, zinc | cutlery, keys, zips, coins, wind and 'brass' instruments, banjo fingerpicks |
Table 1 Important alloys of copper and their uses.
 |
| Figure 9 This saxophone is made from a copper alloy. By kind permission of Matthew Waddington. |
The Euro coins use copper in four different ways. The 1 to 5 cent coins are steel with a copper coating. The 10 to 50 cent coins are made of Nordic gold (89% copper with aluminium, zinc and tin). The 1 and 2 Euro coins contain two alloys. The inner part of the coin with a gold colour is made of 75% copper with zinc and nickel and the outer part, a silver colour, is an alloy of 75% copper and 25% nickel.
Research has shown that bacteria cannot survive long on a copper surface, and one of the advantages of using copper alloys for wind or 'brass' musical instruments is that they are less prone to growth of moulds and bacteria, despite regular exposure to moist, warm, bacteria-laden breath. This effect is apparent if the proportion of copper in the alloy is 65% or greater, and is based on the ability of copper ions to disrupt electron transport in the respiration systems of bacteria cells. Copper can also bind to the phosphate groups in the cell DNA, causing the double-helix to unravel.
Date last amended: 2nd October 2016