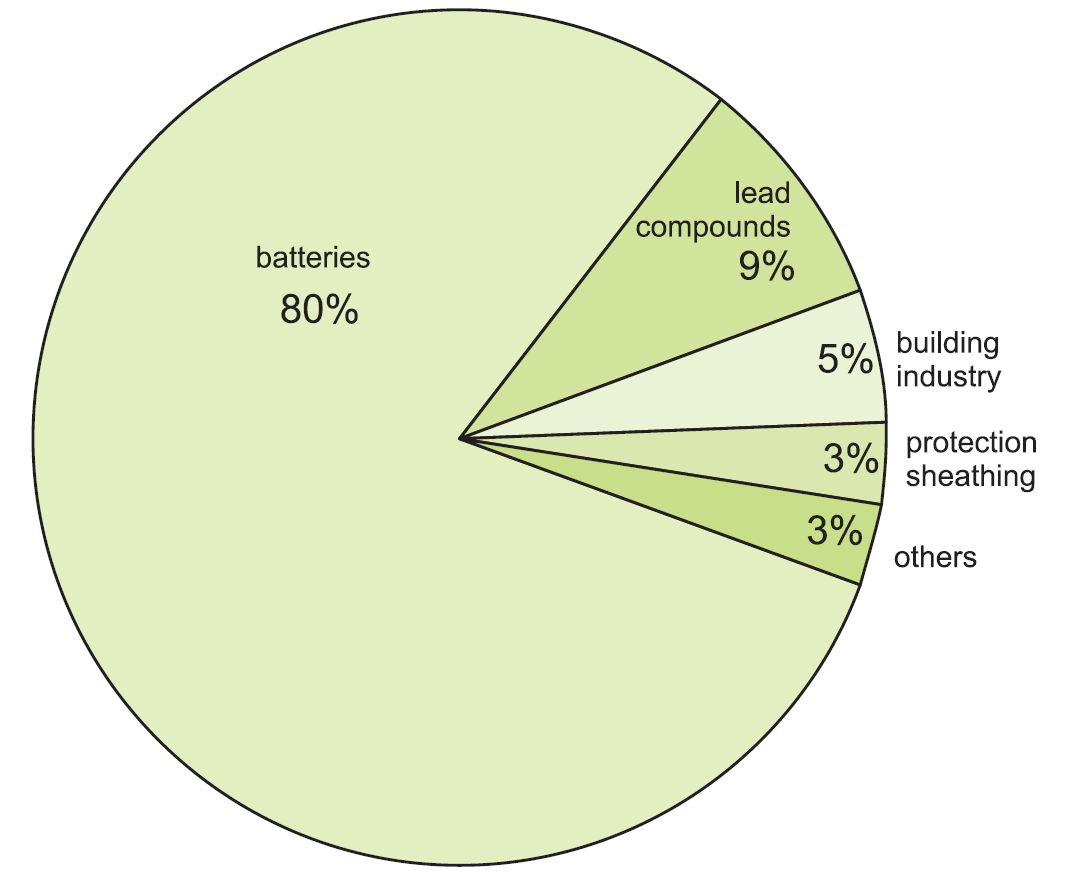
Uses of lead
Over 80% of all lead produced ends up in lead-acid batteries, with lead metal as the cathode and lead(IV) oxide as the anode. In addition to starter batteries for road vehicles, these are also used for zero emission and hybrid vehicles, back-up power (for example for computers and telephone systems), and energy storage in remote power applications.
Figure 1 Uses of lead.
The rest is used in a wide range of applications:
- radiation screening, either as metal sheet (when using X-ray and other powerful radiation equipment) or as lead compounds in glass to protect from radiation (as in television tubes and in medical equipment)
- as lead compounds added to poly(chloroethene) (PVC) as a stabiliser (where durability is important)
- in roofing (for example, for flashings on roofs)
- pipes and lining of vessels in chemical plants (lead is no longer used for domestic water pipes)
- protection of underground or underwater power cables
Lead is also used in a variety of alloys with steel, brass and aluminium (to improve machinability) and with tin for soldering.
Annual production (Primary lead)
These figures are for primary production from the ore and do not include secondary production from recycled material.
| World | 4.7 million tonnes |
| China | 2.3 million tonnes |
| Australia | 633 000 tonnes |
| U.S. | 385 000 tonnes |
| Peru | 300 000 tonnes |
| Mexico | 240 000 tonnes |
| India | 130 000 tonnes |
Data from:
U.S. Geological Survey, Mineral Commodity Summaries, 2016.
Manufacture of lead
About half of the world's refined lead comes from lead ore, the other half coming from secondary (scrap) lead sources. China and Australia have the largest amounts of lead ores and are also the largest primary producers of the metal. Canada, US and Peru have smaller but significant amounts of the ores.
|
The most important lead ore is galena (PbS). Other important ores, such as cerrusite (PbCO3) and anglesite (PbSO4), may be regarded as weathered products of galena and are usually found nearer the surface.
The primary manufacture of lead involves three stages:
a) ore concentration
b) smelting
c) refining
(a) Ore concentration
Lead and zinc ores (usually sphalerite, ZnS) often occur together and may also contain silver, copper and gold. The ore has first to be separated from clays and other silicates ('gangue') after which the lead ore and the zinc ore are separated.
The process used is froth flotation, a succession of stages each delivering a greater concentration of the lead ore. The ore, containing gangue (which typically contains 3-8% lead) is ground with water to a particle size similar to that of fine sand (<0.25 mm). This is then mixed with water and a frothing agent (a detergent) and is violently agitated by air to form a fine suspension with a froth of bubbles on the top. The process is carried out in a series of tanks. As the lead and zinc minerals are less readily wetted than the gangue, they adhere to the air bubbles which are carried to the surface. The rock particles sink and the lead and zinc ores are skimmed off.
Then the lead ore is separated from the zinc ore. A chemical called a depressant is added which is soluble in water (for example, zinc sulfate) and the zinc ore sinks and the lead ore is skimmed off. Later, a chemical such as copper(II) sulfate is added and the zinc ore now floats and is skimmed off.
The lead ore concentrate from the flotation tanks now contains about 50% lead and ca 0.1% of silver, a relatively small but valuable amount.
(b) Smelting
Smelting is usually a two-stage process as described here, although single-stage methods with lower energy use and emissions are also used.
After mixing with limestone the filtered concentrated ore is roasted in air or oxygen-enriched air on a moving belt. Most of the sulfide is converted to lead(II) oxide:
Sulfur dioxide gas can then be cleaned and used to make sulfuric acid.
The lead(II) oxide is heated and made into lumps, a process known as sintering. The lumps (the sinter) are crushed and sorted to a suitable size for subsequent treatment in a blast furnace, similar in construction to those for making iron but smaller.
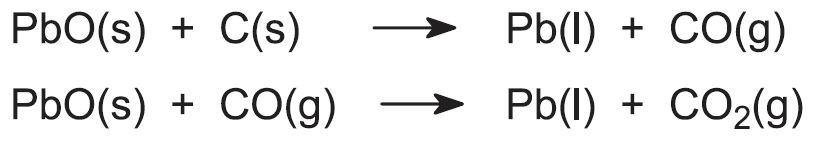
The graded sinter (each lump about the size of a fist) is mixed with approximately 7% of its mass in coke and limestone. The coke is added for two purposes, one as the reducing agent and the other as a source of heat when it reacts with the air which, as in the manufacture of iron is pumped into the furnace. The limestone provides material for the flux containing the impurities, the slag. The mixture is fed into the top of the blast furnace, and the lead(ll) oxide is reduced to molten lead. Carbon and carbon monoxide, produced from the coke, are the reducing agents:
The molten lead is tapped off from the base of the furnace and either cast into, typically, 4 tonne ingots or put into a 'holding kettle' which keeps the metal molten for the refining process.
The product contains about 99.5% lead, the remaining 0.5% being mostly antimony and silver with smaller amounts of other metals, including gold. Because it contains silver and gold at this stage, the lead is referred to as bullion lead.
The fluxes form a molten slag of metal oxides and silicates which floats on top. The slag contains much of the zinc remaining in the ore and is treated later to produce metallic zinc.
Smelting can also be done using a lance through which oil and oxygen-enriched air pass at high speed forming turbulent conditions. There are several such processes operating under names such as Isasmelt, Ausmelt and Sirosmelt. The one stage, Isasmelt, process is discussed at the end of this unit.
(c) Refining
The bullion is heated to just above its melting point. Solid copper and copper sulfide rise to the surface and are skimmed off.
Arsenic is then removed by stirring the lead with an air blast and skimming off the resulting slag which contains arsenic oxides.
Silver is removed by a technique known as the Parkes process. Approximately 2% zinc is added to the lead and a silver-rich zinc crust forms and is removed. More zinc is then added at 740 K (its mp is 693 K) and the bath cooled to just above its melting point. During cooling a solid silver/zinc crust separates, rises to the surface and is removed continuously.
The Parkes process depends on the following:
- lead and zinc are almost immiscible just above their melting points
- silver is much more soluble in zinc than in lead
- silver/zinc alloys have higher melting points than pure zinc.
At 863 K zinc is removed by transferring the lead to a 'dezincing kettle'. At this stage the desilvered lead contains about 0.6% zinc and 0.0004% silver. Dezincing is carried out by vacuum distillation at 860 K when the zinc vaporises.
Finally, all traces of antimony and zinc are removed by mixing sodium hydroxide into molten lead at 760 K (its melting point is 600 K). The resulting sodium zincate and antimonate form a skin on the surface of the molten lead which is skimmed off. The refined lead is of 99.99% purity.
Secondary production
Globally, over 50% of the lead used annually comes from recycled sources. Indeed over 90% of lead used in the US is from recycled metal.
| World | 5.5 million tonnes |
| China | 1.5 million tonnes |
| U.S. | 1.1 million tonnes |
| India | 340 000 tonnes |
| Germany | 290 000 tonnes |
| Mexico | 205 000 tonnes |
| Brazil | 188 000 tonnes |
Data from:
International Lead Association, 2012.
Lead for recycling may be in the form of scrap metal (roofing sheet, for example), or compounds of lead, such as the pastes from lead-acid batteries. Clean metallic lead can be melted and refined directly, but compounds and lead alloys require smelting, using processes similar to those used with lead ores.
Two-stage process
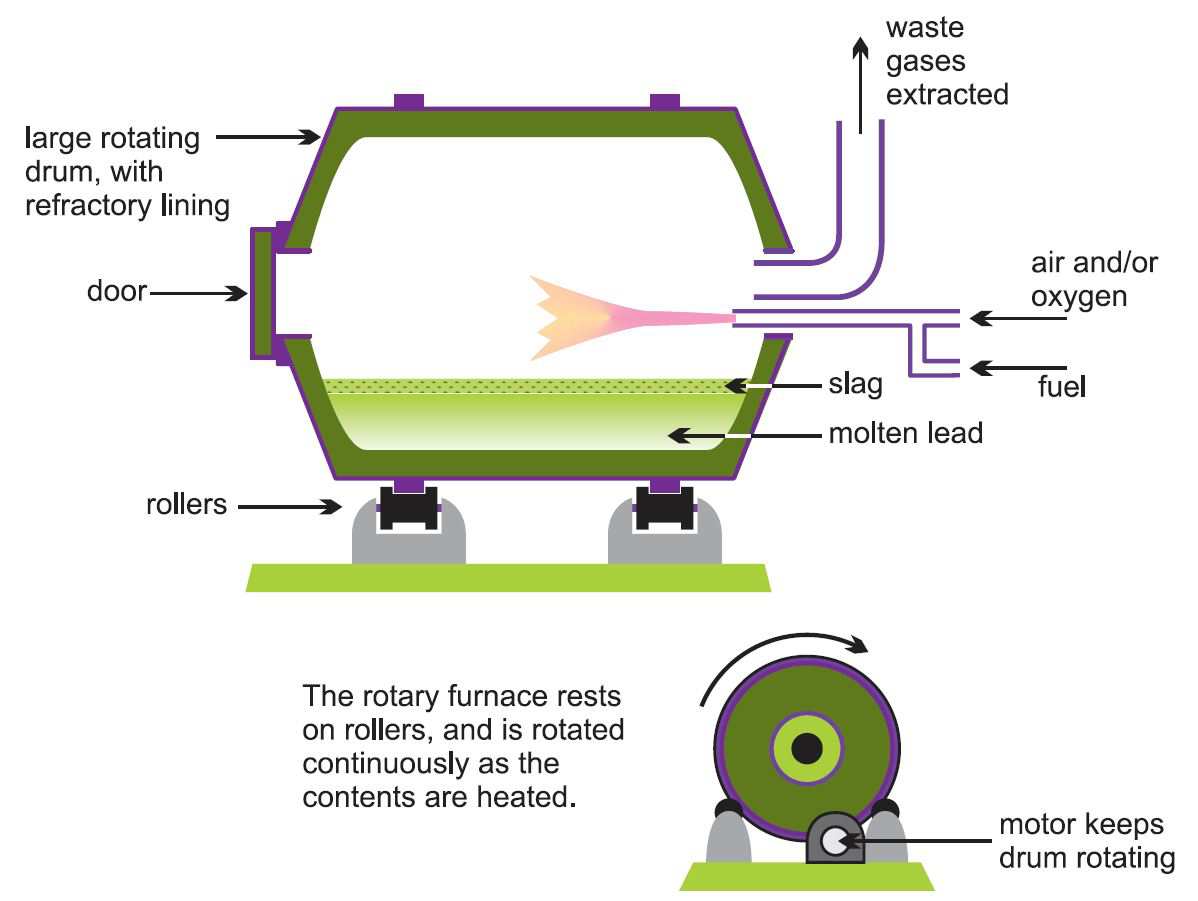
In the two-stage process for secondary production, smaller rotary or reverberatory furnaces (Figure 3) are used rather than larger blast furnaces as this gives better control of the quality of the lead. Rotary furnaces can accept lead in almost any form, and can make use of many different carbon sources (natural gas, oil and coke) for reduction.
The first stage uses very little reducing agent and once the furnace is heated any metallic lead melts and can be tapped off after a few hours. This will have a high purity as other materials, including lead compounds, remain in the slag. Further scrap is added and the process is repeated until sufficient slag has accumulated for the second stage.
The second stage involves the reduction of the slag using a carbon-based reducing agent. Sodium carbonate ('soda ash') or calcium carbonate is also added as a 'flux' to help form the slag of impurities. Lead oxides, lead sulfate and any antimony oxides are reduced, and the result is 'antimonial lead', which may also contain some bismuth and silver. The antimony (2-5%) gives the lead greater strength.
Figure 3 Illustrating a rotary furnace, used here in the secondary production of lead.
One-stage process
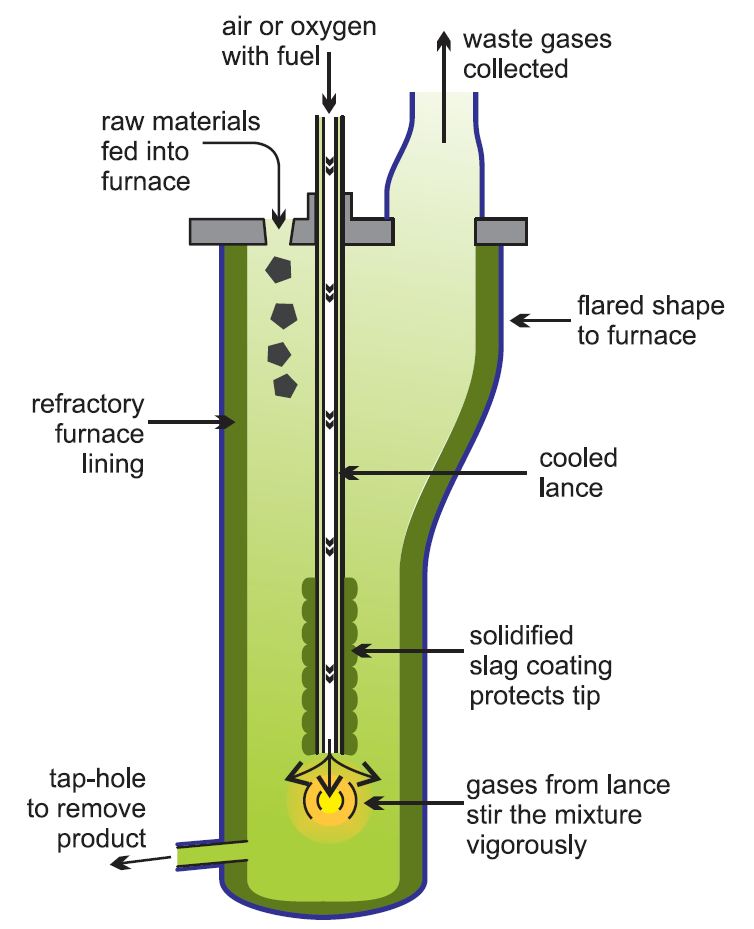
The Isasmelt process, Figure 4, is an example of one of the most modern methods of secondary lead production in which a one stage process is used, particularly for processing the paste from batteries. This is fed into a furnace and melted using a lance through which a mixture of oil and oxygen-enriched air are fed. It is more economic to use oxygen-enriched air, rather than air as this increases the reaction rates and means that smaller chemical plants can be used and fuel cost are reduced. Further it makes it easier to ensure that no gases such as sulfur dioxide are lost and pollute the atmosphere. Oxygen plants are constructed on the site.
Figure 4 The production of secondary lead using the Isasmelt process.
For the next 36 hours more paste, together with coal as a reducing agent, is fed into the furnace at about 1250 K and lead with a purity of 99.9% is tapped off every few hours.
Later, fluxes are added and the temperature is raised to 1500 K. This reduces the slag, which again results in antimonial lead.
The Isasmelt process has higher thermal efficiency, and the remaining waste slag has a lower residual lead content.
The process is also used for primary lead production.
Date last amended: 26th September 2016